BE ADVISED:
The beamlines at Sector 11 are working hard to transition back to operations following the extensive work performed by its group members alongside the APS Upgrade staff. 11-BM has successfully completed its Shielding Verification and has begun Technical Commissioning. 11-ID has started its shielding verification and will transition into Technical Commissioning in late February. We expect to return to Operations soon. Please check back frequently for updates.
Overview
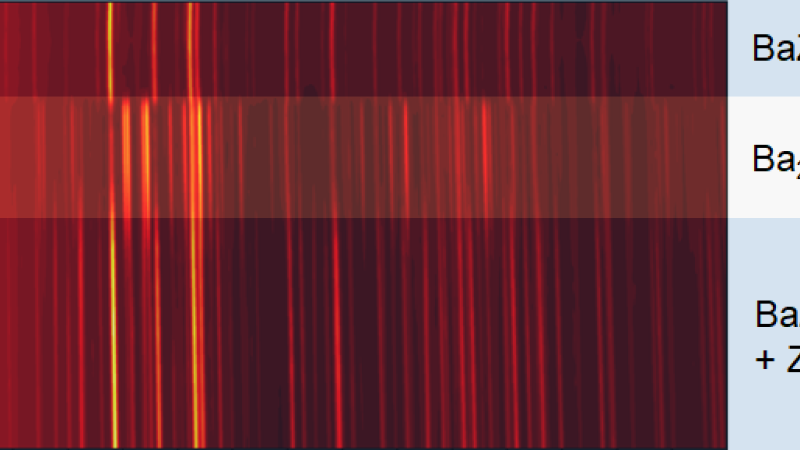
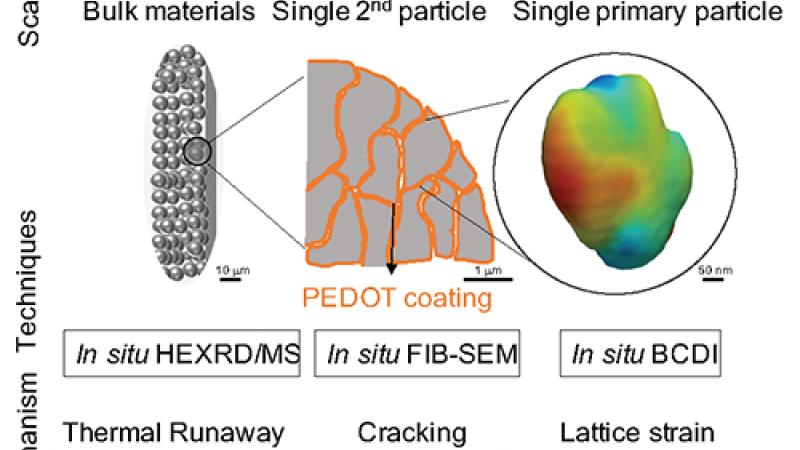
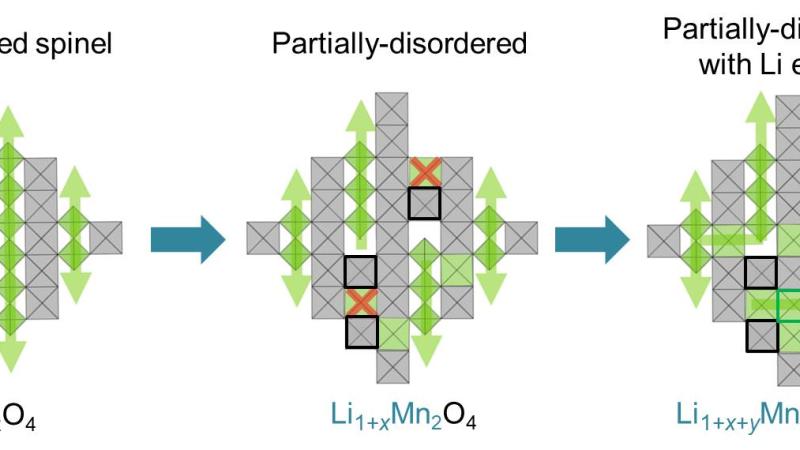
High-energy X-ray diffraction (XRD) is a bulk structure characterization technique widely used in materials research taking advantage of the high penetration power into materials, large accessible q-range, and small correction factors. In situ measurements study materials under various conditions. Operando studies are essential for insights into structural changes in functional materials during operation with the goal of improving their performance. XRD can resolve phase identities and other features, like particle size, micro-strain, structural defects, etc. The beamlines operated by the Structural Science (SRS) group offer a wide range of in situ and operando powder XRD and total scattering measurement capabilities with sub-second temporal resolution. The beamlines operate in x-ray transmission geometry with monochromatic beams and area detectors. In addition, 11-BM utilizes an array of analyzer crystals and scintillators to achieve the highest resolution. Most recent developments include an in-line Raman system at 17-BM and 11-ID-B that allows simultaneous measurement of XRD/PDF and Raman spectroscopy data under controlled temperature and gas environments. 11-ID-B enables studies of surfaces and interfaces with an optional 1D vertical focusing on the order of a few micrometers. 11-ID-B utilizes a hexapod positioning stage to allow highly accurate control of sample orientation, enabling surface and interface scattering. 11-ID-C employs a heavy-load hexapod providing similar capabilities for heavy equipment up to 250 kg. The high-resolution 11-BM beamline now has the option to switch to an area detector for rapid checks on the sample status. The group has a strong emphasis on battery research, maintaining an electrochemistry facility which is accessible 24/7 for all APS users for cell preparation. This eChem Lab offers standard lab equipment plus two Argon atmosphere glove boxes with one dedicated for Sulphur containing materials.
11-BM, 11-ID-B and 17-BM all offer Mail In services.
After the upgrade in 2024, 11-ID-D will become the flagship for structural science offering the combination of high-energy x-ray total scattering including small angle scattering with strong beam focusing capabilities in the x-ray regime between 26 keV to 120 keV.
| 11-BM | ||
 | 11-BM is dedicated to high-resolution powder diffraction measurements. The instrument operates over the energy range from 25-35 keV, and combines a sagittally focused monochromator with multiple Si crystal analyzers to achieve exceptional resolution and sensitivity. The beamline offers a unique mail-in service for rapid access, and supports on-site user experiments for non-routine powder diffraction measurements. | Technical Specifications |
| 11-ID-B | ||
 | 11-ID-B is dedicated to Pair-Distribution-Function (PDF) measurements with area detectors. The instrument operates at high X-ray energies (58.6 keV, 86.7 keV) and is optimized for High throughput measurements and non-ambient / in-situ measurements. Typical configurations may involve a sample changer, cryostream, compact furnace/flow cell, and single-crystal diffuse scattering (under development). Now accepting Mail In proposals. More information can be found on the mail-in wiki. | Technical Specifications |
| 11-ID-C | ||
 | 11-ID-C is used for scattering studies at extreme conditions. The high energy X-ray beam (105.7 keV) is highly penetrating and allows a wide coverage of reciprocal space over a small angular scattering range. This is particularly advantageous for experiments that require bulky sample environments (e.g. loadframes, 3D printed bulk materials, industrial batteries). | Technical Specifications |
| 11-ID-D | ||
 | 11-ID-D will offer a wide range of experimental approaches with highest available flux. Two dimensional focusing will be available and adaptable energy resolution. A q-range from 0.01 A-1 to 40 A-1 will be covered. The photon energy will cover the energy range from 26 to 120 keV. A secondary detector for spectroscopic imaging will expand the multimodal approaches. | Technical Specifications |
| 17-BM-B | ||
 | 17-BM-B is dedicated to rapid acquisition powder diffraction experiments using an area detector, where moderate resolution data can be obtained in fractions of seconds. The versatile set-up can accommodate a wide range of sample environments and is well suited to parametric/in situ/in operando measurements. Standard configurations include a sample changer, cryostream, compact furnace/flow cell, compact pressure cells (<10 GPa, under development). Now accepting Mail In proposals. More information can be found on the mail-in wiki. | Technical Specifications |
| eChem Lab | ||
 | The eChem lab is equipped with a state-of-the-art Ar-atmosphere glovebox with fridge/freezer unit, and small and large antechambers. The glovebox is supplied with Li and Na metals, standard electrolytes, coin cell crimping tools and accessories needed to assemble battery cells. The lab is also equipped with three mobile 8-channel MACCOR® battery cyclers capable of running independent experiments, a single-channel CH Instruments® potentiostat, a vacuum oven, balances, bench space and standard laboratory consumables. | Details |