Thermoelectric materials, which convert heat into energy, are challenging to design because of the unusual set of interrelated properties required for high efficiency: a high Seebeck coefficient (a measure of the induced thermoelectric voltage in response to a temperature difference across the material), high electrical conductivity, and low thermal conductivity. One family of materials that has shown great potential in this area is binary zinc antimonides, especially in the temperature range of 400 to 600 K. Their promising properties have spurred interest in investigating systems based on zinc and antimony, many of which have also shown promise as thermoelectric materials.
For example, researchers have discovered a type-I clathrate where the zinc and antimony framework atoms form cages around cesium ions. This material is electron-balanced and follows the electron-counting principle for type-I clathrates, which postulates that all framework atoms require four valence electrons since these atoms are tetrahedrally coordinated. Replacing the cesium atoms in this clathrate with an alkaline earth element, such as barium, would require adjustment of the zinc-antimony ratio to accommodate an excess eight electrons. Although such a compound is theoretically possible according to electron counting, it had yet to be synthesized.
Researchers from Iowa State University and the University of California-Davis accomplished this feat, using beamline 17-BM at the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, to provide evidence of two “hidden” compounds that can only be synthesized in a narrow temperature range. The findings emphasize the power of the primitive approach of electron counting in predicting compounds and the potential of these two new compounds in thermoelectrics.
To balance electrons, the researchers aimed to create a compound from barium, zinc and arsenic. By combining these elements in stoichiometric ratios and heating them to 900 °C, then annealing at a lower temperature, the researchers successfully obtained single crystals of this material. The single crystals allowed researchers to study the crystal structure, which they described as clathrate-like, composed almost entirely of anion cages made of zinc and arsenic filled with barium cations. They used the same approach to produce a compound in a different ratio. However, samples of both compounds had persistent admixtures that prevented study of their thermoelectric properties.
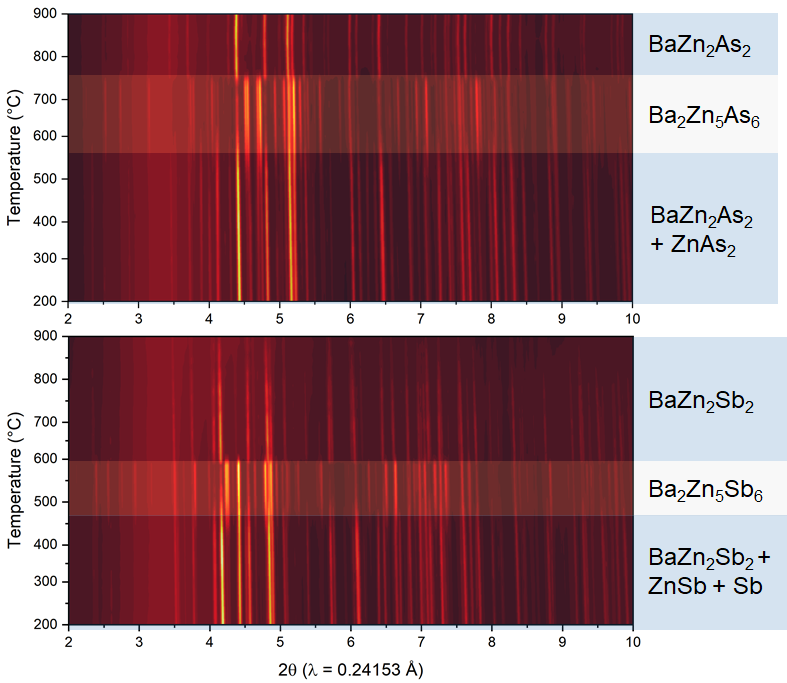
To learn how these compounds formed and explain the admixtures, the researchers performed powder X-ray diffraction at the APS beamline. Their findings show that the main product at the start of the experiment was BaZn2Pn2. However, when heating began, this compound was transformed into the respective Ba2Zn5Pn6 phase. This was the dominant phase for about 100 °C before transforming back into BaZn2Pn2 and presumably a melt of Zn-Pn2. Upon cooling, Ba2Zn5Pn6 never reformed, even as a minor product.
These experiments revealed the most probable reason why Ba2Zn5Pn6 had never been discovered before now, despite heavy exploration of ternary systems such as this one: The small temperature range in which these compounds are accessible is crucial for these materials to form in a single phase.
Theoretical calculations showed that this material is thermodynamically stable only in this small temperature range; upon further heating, this phase decomposes. The researchers confirmed these findings using a method called differential scanning calorimetry.
Using a different synthesis approach that took advantage of precursor compounds, as well as the vital information from the 17-BM experiment, the researchers produced enough of this material to study its thermoelectric properties. Both Ba2Zn5As6 and Ba2Zn5Sb6 displayed high Seebeck coefficients and low thermal conductivity, but they also had relatively high resistivity, a barrier to their use as thermoelectric materials. The researchers speculate that native defects in these materials could negatively affect their resistance, which could be reduced with various approaches, such as doping.
The scientists suggest that if the conductivity of these materials can be improved, they could be promising new thermoelectric materials. – Christy Brownlee
___________________________________________________________________________
See: P. Yox1,2, F. Cerasoli3, A. Sarkar1,2, V. Kyveryga1, G. Viswanathan1,2, D. Donadio3, K. Kovnir1,2, “New Trick for an Old Dog: From Prediction to Properties of “Hidden Clathrates” Ba2Zn5As6 and Ba2Zn5Sb6,” J. Am. Chem. Soc. 145, 8, 4638-4646 (2023)
Author affiliations: 1Iowa State University; 2Ames National Laboratory; 3University of California Davis.
This research was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Science and Engineering, grant DE-SC0022288. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357. The PPMS instrument used for property measurements was supported by Ames National Laboratory, U.S. Department of Energy, which operates under the contract DE-AC02-07CH11358.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
