Given the unending interest in portable electronics of all kinds, there’s been an upsurge in research on high-energy-density cathode materials for rechargeable lithium-ion (L-ion) batteries. State-of-the-art layered Li(Ni,Mn,Co)O2 cathode materials provide good energy and power density for many applications; however, potential further improvement in these materials is limited. Additionally, as the lithium-ion industry continues to grow, so does the use of cobalt or nickel, straining scarce metal resources. To sustain this continued growth, development of new cathodes with high energy densities made from Earth-abundant elements will be necessary. Recent research has identified some promising energy-dense materials, such as Li-rich layered oxides and cation-disordered rock-salt-type cathodes; however, these materials don’t have the rate capability (the rate for charge/discharge) high enough to make rechargeable batteries practical. To create both dense and fast energy storage will require an overhaul of the cathode material’s makeup and structure. Toward that end, users of the U.S. Department of Energy’s (DOE’s) Advanced Photon Source (APS) report two new lithium-ion cathode materials with a structure that shares features with those of gemstones known as spinels but with a significant amount of disorder. These inexpensive materials exhibit both high energy density and high rate capability, giving them extraordinary potential as cathodes in lithium-ion batteries.
The research team, including members from the University of California Berkeley, Lawrence Berkeley National Laboratory, Oak Ridge National Laboratory, and the University of California, Santa Barbara, and Argonne National Laboratory sought a face-centered-cubic anion framework for achieving dense energy storage because it’s a close-packed crystalline arrangement. For high power density, the cations—including lithium and transition metal ions—need to be optimally positioned within this anion framework.
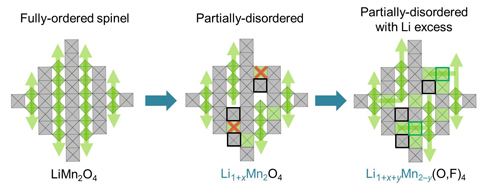
Previous work has suggested that a spinel-like cation order—which follows the structure of spinel gemstones and places divalent and trivalent cations in some or all of the octahedral or tetrahedral sites in the crystal lattice—can help lithium ions move efficiently in the framework. This arrangement promotes lower electrostatic repulsion between lithium and other cations compared to alternate structures. However, other spinel-like materials have been unsuitable for cathodes because they couldn’t reliably cycle over low-voltage plateaus between discharged and charged states.
To overcome this issue, the researchers created two oxyfluorides with partial spinel-like order: Li1.68Mn1.6O3.7F0.3 (LMOF03) and Li1.68Mn1.3O3.4F0.6 (LMOF06) (Fig. 1).
After synthesis of these materials through mechanochemical alloying, the scientists characterized their structures by performing ex situ synchrotron diffraction using the X-ray Science Division (XSD) Structural Science Group’s 11-1D-B beamline at the Argonne National Laboratory APS; operando x-ray absorption near edge spectroscopy experiments on the XSD Spectroscopy Group’s 20-BM beamline at the APS; mapping of resonant inelastic x-ray scattering measurements at the iRIXS end station on beamline 8.0.1 of the DOE’s Advanced Light Source (ALS) at Lawrence Berkeley National Laboratory; and neutron powder diffraction using the DOE’s Spallation Neutron Source (SNS) at Oak Ridge National Laboratory on the Nanoscale Ordered Materials Diffractometer. The APS, ALS, and SNS are Office of Science user facilities.
Their results showed a spinel-like structure with a pseudo face-centered-cubic anion framework. Both materials also displayed very high specific energies (energy per unit mass) and discharge rates—for example, samples of LMOF03 showed specific energies greater than 1,100 Wh kg-1 and discharge rates up to 20 A g-1.
These qualities are possible, the authors explain, because of the materials’ unusual stoichiometry. Unlike ordered spinels, which have a 3:4 ratio of cations to anions, the new materials’ ratio is 3.28:4. They also have a lithium excess compared to the ideal stoichiometry of LiM2O4, where M is a non-lithium metal ion, because Li is partially substituted for Mn. The excess lithium decreases electrostatic repulsion for a larger capacity and better lithium transport kinetics. The positive charges from this lithium excess are balanced by negatively charged fluorine, which in turn improves the ability of the material to cycle between charge and discharge. In addition, this cation overstoichiometry induces partial disorder in the metal ions, which overcomes the problems other spinel-like structures had with cycling over low-voltage plateaus.
Interestingly, further experiments show that half of the capacity in LMOF03 comes from reversible oxygen redox, a phenomenon observed in lithium-rich layered Ni-Mn-Co oxides and disordered rock-salts but is uncommon in spinel-like cathodes.
The authors suggest that these findings highlight the potential for designing high-performance and resource-efficient cathode materials that lie somewhere between fully ordered and disordered compounds. ― Christen Brownlee
See: Huiwen Ji 1,2, Jinpeng Wu 2, Zijian Cai1, 2, Jue Liu3, Deok-Hwang Kwon1, 2, Hyunchul Kim2, Alexander Urban4, Joseph K. Papp1, Emily Foley5, Yaosen Tian1, 2, Mahalingam Balasubramanian6, Haegyeom Kim2, Raphaële J. Clément5, Bryan D. McCloskey1,2, Wanli Yang2, and Gerbrand Ceder1,2, “Ultrahigh power and energy density in partially ordered lithium-ion cathode materials,” Nat. Ener. 5, 213 (March 2020). DOI: 10.1038/s41560-020-0573-1
Author affiliations: 1University of California, Berkeley, 2Lawrence Berkeley National Laboratory, 3Oak Ridge National Laboratory, 4Columbia University, 5University of California, Santa Barbara, 6Argonne National Laboratory
Correspondence: *gceder@berkeley.edu
This work is supported by the Umicore Specialty Oxides and Chemicals and the Assistant Secretary of Energy Efficiency and Renewable Energy, Vehicle Technologies Office of the U.S. Department of Energy (DOE) under contract no. DE-AC02-05CH11231 under the Advanced Battery Materials Research (BMR) Program. Recent characterization work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Vehicle Technologies Office, under the Applied Battery Materials Program, of the U.S. DOE under contract no. DE-AC02-05CH11231. Work at the ALS is supported by the Director, Office of Science-Basic Energy Sciences, of the U.S. DOE under contract no. DE-AC02-05CH11231. Research conducted at the Nanoscale Ordered Materials Diffractometer Beamline at Oak Ridge National Laboratory’s Spallation Neutron Source is sponsored by the Scientific User Facilities Division-Basic Sciences of the U.S. DOE. Work at the Molecular Foundry at Lawrence Berkeley National Laboratory is supported by the Office of Science-Basic Energy Sciences of the U.S. DOE under contract no. DE-AC02-05CH11231. H.J. acknowledges support from the Assistant Secretary of Energy Efficiency and Renewable Energy, Vehicle Technologies Office of the U.S. DOE, under contract no. DE-AC02-05CH11231. J.K.P. also acknowledges support from the National Science Foundation Graduate Research Fellowship under contract no. DGE-1106400. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the U.S. DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
