 Light is an important feature of the natural world. Many organisms have developed sophisticated systems to detect light and then convey signals to sensory systems that respond. This can be achieved through coupled systems that contain both a light-sensing chromophore and a protein that passes on the information via protein conformational changes to other domains or proteins in the system.
Light is an important feature of the natural world. Many organisms have developed sophisticated systems to detect light and then convey signals to sensory systems that respond. This can be achieved through coupled systems that contain both a light-sensing chromophore and a protein that passes on the information via protein conformational changes to other domains or proteins in the system.
However, these reactions work on very fast timescales and not much is known about the structural intermediates that are involved. This information is important for understanding how these systems work and could be useful for applications such as the design of light-activated cellular sensors for research or medical treatments.
In a recent publication, a collaborative team from the Korean Advanced Institute of Science and Technology (KAIST), the Korean Center for Advanced Reaction Dynamics, and the University of Chicago reported on their findings from work conducted at the University of Chicago’s BioCARS 14-ID-B beamline at the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory. Their results provide new structural and mechanistic insights to further illuminate this process.
The research focused on a light-sensing protein from the common oat plant, Avena sativa, called AsLOV2, a member of a superfamily of light-activated proteins that contain the light-oxygen-voltage (LOV) domain. These LOV domain-containing proteins detect blue light in the visible spectrum and have a conserved structure composed of five β sheets and four α helices. When blue light activates the chromophore, a covalent bond is formed between the light-sensing molecule and a cysteine amino acid on the protein. This is hypothesized to lead to protein dimerization and other conformational changes that transmit the light signal downstream.
The team used time-resolved X-ray liquidography (TRXL), a sensitive technique that can detect global conformational changes in solution on a millisecond to microsecond timescale, to analyze the light-activated transition of AsLOV2.
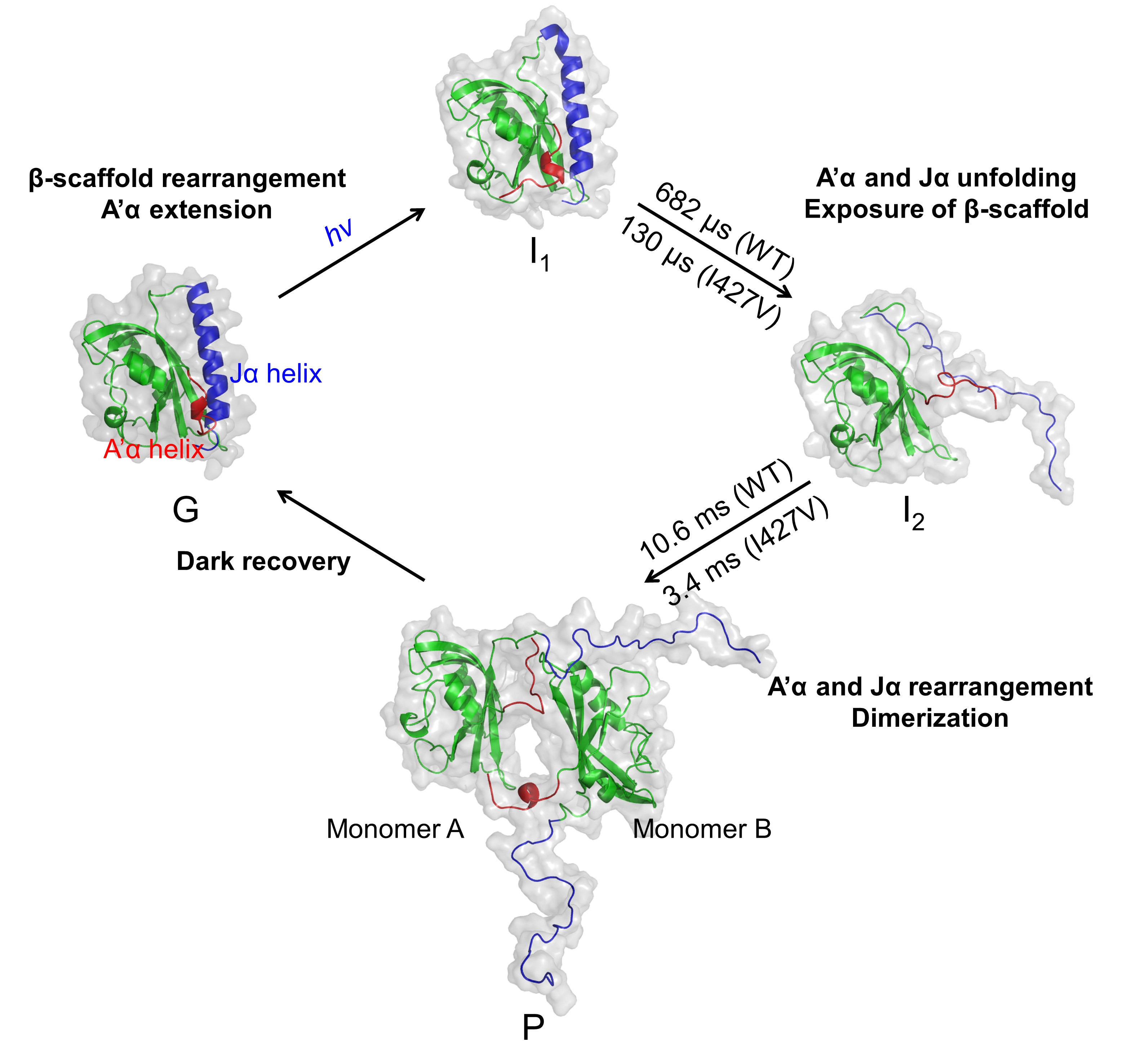
The structure of interest for the work was a piece of the full-length AsLOV2 protein that contained the LOV domain and two helices, A’α and Jα, that are known to be involved in the light-induced dimerization of the protein and downstream signaling. The team used a mutant–type of the protein (I427V) that has a faster recovery rate than the wild–type (WT) protein, facilitating some of the measurements. Kinetic evaluation of the TRXL data showed that light-induced transition of AsLOV2 includes ground (G), first intermediate (I1), second intermediate (I2), and final photoproduct (P) states with associated time constants (WT: 682 microseconds [μs] and 10.6 milliseconds [ms], and I427V: 130 μs and 3.4 ms).
Structural analysis of the TRXL data along with molecular dynamics simulations of possible configurations of the protein based on the crystal structure of the ground (dark) state of the protein suggested that the first intermediate involved minor structural changes consistent with extension of the A’α helix. Modeling for the second intermediate, occurring on a somewhat slower μs timescale, was consistent with unfolding of the Jα helix on the other side of the molecule from the A’α helix.
For the third structure, the low-resolution conformations of the G and P states, generated from small-angle X-ray scattering (SAXS) data, demonstrated that AsLOV2 exhibits light-induced dimerization. Furthermore, the molecular dynamic simulation-aided analysis showed that the dimerization of AsLOV2 occurs as the β-scaffolds of the AsLOV2 monomers, exposed by the unfolding of the A’α and Jα helices, form a dimeric interface.
These main findings show that light activation of AsLOV2 causes the A’α and Jα helices to unfold within microseconds and that this facilitates the exposure of the β-scaffold structure of the LOV2 domain to activate dimerization and form the final product of light activation on a millisecond timescale. These data are consistent with previous data on how AsLOV2 transforms light into a downstream signal and provide support for a model in which light-induced dimerization of the LOV domains facilitates activation of the downstream enzymes responsible for signal transmission. – Sandy Field
_____________________________________________________________________________
See: C. Kim1,2, S.R. Yin1,2, S.J. Lee1,2, S.O. Kim1,2, H. Lee1,2, J. Choi1,2, J.G. Kim1,2, T.W. Kim1,2, S.You1,2, I. Kosheleva3, T. Noh1,2, J. Baek1,2, H. Ihee1,2, “Structural dynamics of protein-protein association involved in the light-induced transition of Avena sativa LOV2 protein,” Nat Commun 15 6991 (2024)
Author affiliations: 1Korean Advanced Institute of Science and Technology; 2Institute for Basic Science; 3University of Chicago.
We thank Yonggwan Kim for his assistance with time-resolved X-ray liquidography experiments. We appreciate Min Byoung Seok and Kyeong Sik Jin, the 4 C SAXS II beamline staff at Pohang Accelerator Laboratory (PAL, Korea), for their assistance with SAXS data collection. This work was supported by the Institute for Basic Science (IBS-R033) granted to H.I. The use of the BioCARS Sector 14 was also supported by the National Institutes of Health, and the National Institute of General Medical Sciences grant R24GM111072. The time-resolved setup at Sector 14 was partially funded through collaboration with P. Anfinrud (NIH/NIDDK) through the Intramural Research Program of the NIDDK.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
