A major facet of transitioning from fossil fuels to green and renewable energy solutions involves the removal, capture and storage of carbon dioxide (CO2) from the environment. One method is by CO2 hydrogenation, which requires a catalyst to spur the reaction, frequently including metal-oxide catalysts in which metal-support interactions (MSIs) play an important role.
Researchers from the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory, Stony Brook University, DOE’s Argonne National Laboratory and several other institutions used a suite of in situ techniques to study the behavior and structural and chemical properties of a Cu@TiOx core@shell catalyst under CO2 hydrogenation. Their work was published in ACS Catalysis.
In a core@shell structure, one type of active system (the core) is encapsulated by a shell of a different material to enhance catalytic performance. These experiments focused on an inverse oxide/metal catalyst configuration using a copper nanowire core with a titanium oxide (titania) shell. Such catalysts have been shown to offer improved stability and activity over the conventional metal/oxide arrangement.
Through the use of an entire range of in situ characterization techniques – including time-resolved experiments with X-ray absorption spectroscopy (XAS), ambient pressure X-ray photoelectron spectroscopy (AP-XPS), environmental transmission electron microscopy (E-TEM), and X-ray diffraction at the 17-BM-B beamline of the Advanced Photon Source, a DOE Office of Science user facility at Argonne – the investigators sought to achieve a comprehensive understanding of the structure and behavior of the Cu@TiOx catalyst under CO2 activation and hydrogenation, a functional picture that cannot be obtained with typical steady state studies.
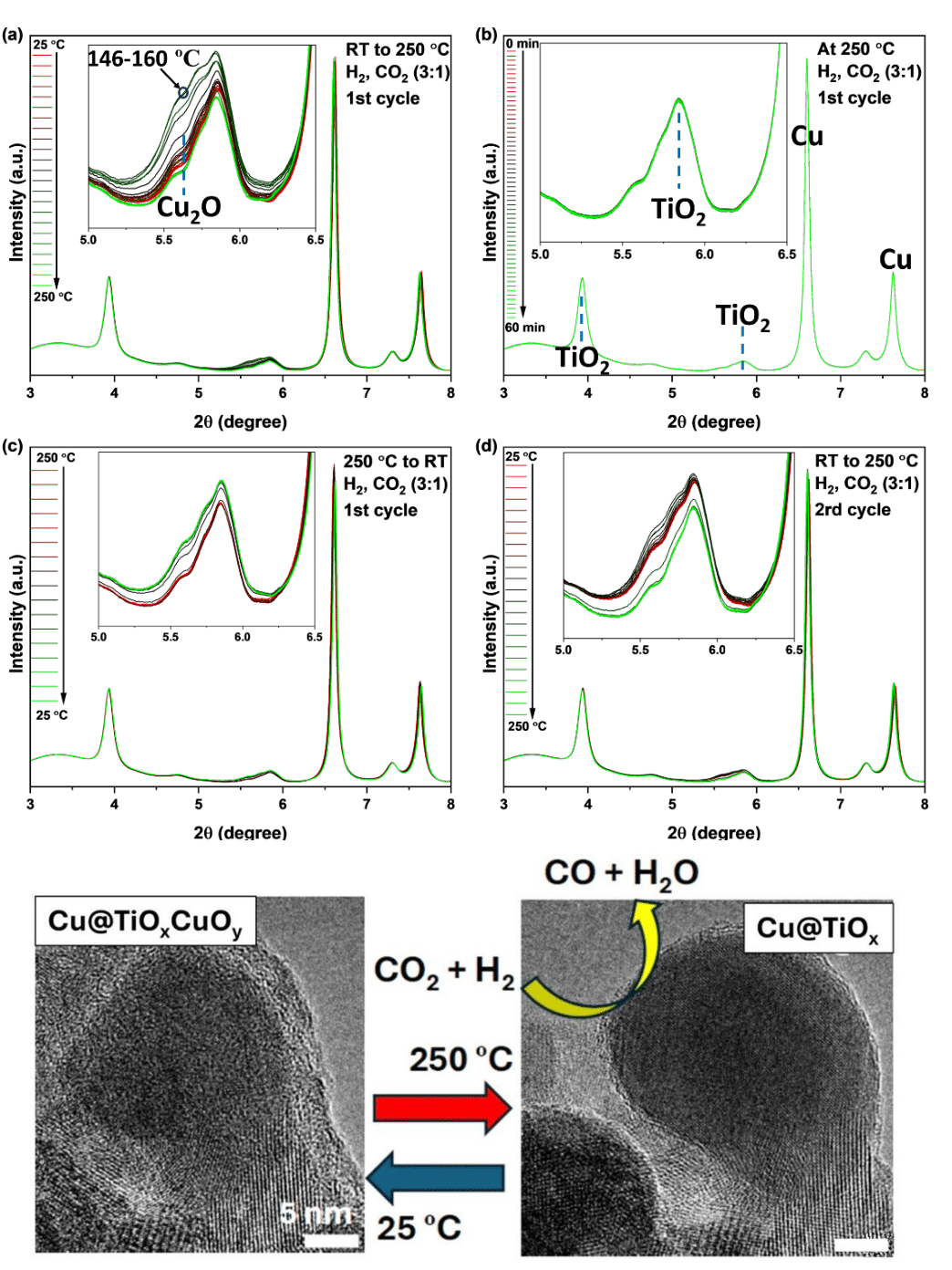
The dynamic characteristics of this catalyst system became immediately evident even during the standard pretreatment used for CO2 hydrogenation, when the H2 pretreatment at temperatures of above 250 degrees Celsius resulted in cracking of the titania shell and migration of Cu particles from the core to the top of the oxide shell. This, along with other configuration changes, was caused by metal-support interactions. The migrating Cu particles are about 20-40 nm in diameter and are speckled with clusters of TiOx and Cu-Ti-Ox. With this altered structure, the system displayed highly dynamic yet wholly reversible catalytic characteristics that were dependent on temperature and chemical environment.
In situ tracking during CO2 exposure at room temperature showed that this configuration changes again, with the TiOx and Cu-Ti-Ox aggregates becoming a layered structure atop the Cu surfaces. Under higher temperatures above 180 degrees Celsius and exposure to H2, this oxide layer vanished, and the only detected reaction product was CO, indicating partial decomposition of CO2 on the surface. The in-situ observations indicate oxidation of Cu and TiOx at room temperatures by CO2 with reduction of CuOx and Cu-Ti-Ox at higher temperatures by H2.
It was clear that neither CO2 nor H2 behaved as passive reactants and that both had significant, yet reversible, effects on the composition and morphology of the Cu@TiOx catalyst. These properties moved toward a dynamic equilibrium when under simultaneous oxidation and reduction in a CO2 and H2 mixture. The work is a new and valuable window into the very active processes involved with a core@shell catalyst under normal operational conditions. Such insights may provide important clues for the design and fabrication of new and more efficient catalytic materials for dealing with the trapping and conversion of CO2. – Mark Wolverton
See: K. Deng1,2, X. Chen3, J. Moncada2, K.L. Salvatore1, N. Rui2, W. Xu4, S. Xiang1, N. Marinkovic5, A.I. Frenkel2,1, G. Zhou3, S.S. Wong1, J.A. Rodriguez1,2, “Observing chemical and morphological changes in a Cu@TiOx core@shell catalyst: Impact of reversible metal-oxide interactions on CO2 activation hydrogenation," ACS Catal. 2024, 14, 15, 11832-11844
Author affiliations: 1Stony Brook University; 2Brookhaven National Laboratory; 3State University of New York; 4Argonne National Laboratory; 5Columbia University
The work done at the Chemistry Division of BNL was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, and Catalysis Science Program under contract No. DE-SC0012704. J.A.R. and J.M. received funding from a DOE Office of Science Distinguished Scientist Fellow Award. X.C. and G.Z. acknowledge the support by the National Science Foundation (NSF) under DMR 1905422. A.I.F. acknowledges support of XAFS data analysis by the U.S. DOE, Office of Science, Office of Basic Energy Sciences grant no. DE-SC0022199. The Cu@TiOx core–shell nanowires, that inspired the current work, were initially produced in SSW’s laboratory, supported by the U.S. National Science Foundation under Grant No. CHE-1807640. This research used resources of beamlines 7-BM (QAS), 28-ID-2 (XPD), and 23-ID-2 (IOS) of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. The QAS beamline operations were supported in part by the Synchrotron Catalysis Consortium (U.S. DOE, Office of Basic Energy Sciences, Grant No. DE-SC0012335). This research used resources of beamline 17-BM at the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. The morphology of the catalyst was studied at the Electron Microscopy Facility (FEI Titan 80-300 and FEI Talos 200×) of the Center for Functional Nanomaterials (CFN), which is a U.S. Department of Energy Office of Science User Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. The authors are grateful to D. Zakharov for his help with some of the E-TEM and EELS studies.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
