Metallic glasses (MGs) are alloys that possess an amorphous (disordered) structure instead of a crystalline lattice. This jumbled atomic arrangement often yields materials with exceptional properties, for instance very high yield strength and toughness. These exceptional features have led to the incorporation of MGs into advanced biomedical implants, superior sports equipment, energy-saving electrical devices, and many other applications.
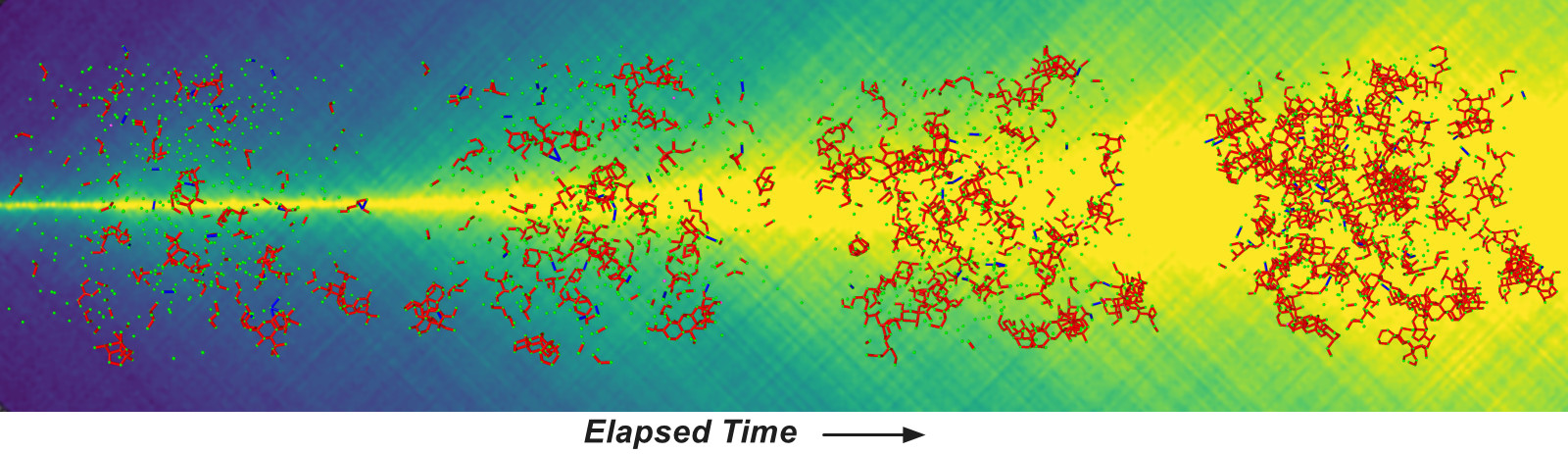
Unfortunately, the disordered structure of MGs inevitably leads to their atoms migrating over time, which can seriously degrade their superior properties. For years scientists have investigated the complex structural rearrangements that occur within metallic glasses, but important details of this dynamic process remain obscure. In this study, researchers measured atomic-level movements in a metallic glass over the unprecedented time span of nearly 3½ days, using X-ray photon correlation spectroscopy (XPCS) performed at beamline 8-ID-E of the Advanced Photon Source (APS). The APS is a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory.
The extremely high-resolution XPCS measurements, recorded continuously over the entire experiment, provide new information about MG aging. For instance, the experiment revealed long stretches of robust structural changes punctuated by periods of minimal internal movements. This study demonstrates the feasibility of long-duration XPCS observations of metallic glasses, while also providing important new insights into their long-term internal dynamics.
Ordinary alloys such as bronze, brass and steel are mixtures of different metals (and often small amounts of non-metals) that readily form an orderly lattice when cast or forged. In contrast, the disorderly structure of metallic glasses is difficult to obtain since they only form under special conditions.
The first metallic glasses were created in the 1960s via extremely quick quenching, at cooling rates of thousands of degrees per millisecond. This extreme cooling protocol meant that only wires or thin ribbons could be formed. Eventually scientists developed thicker MGs (called bulk metallic glasses) that avoided rapid quenching in favor of, for example, employing numerous atomic elements that encompass a large range of sizes, a strategy which makes it difficult to form an orderly crystalline structure.
Immediately after a metallic glass solidifies, its atoms begin rearranging into a lower energy configuration. This energy-driven structural rearrangement has been studied by materials scientists for decades and has shown, for instance, that atoms migrate in groups, or clusters, in a process called cluster dynamics. However, a full characterization of the complex mechanisms driving the microscopic movements in MGs is still lacking, including whether those structural changes eventually settle upon a more-or-less uniform behavior.
The bulk metallic glass examined in this study was comprised of five distinct elements that formed a zirconium-titanium-copper-nickel-aluminum alloy. This alloy was annealed (heated) to a temperature of 668 Kelvin (about 743 degrees Fahrenheit), which is just below the glass transition temperature, where the metallic glass softens to a jelly-like consistency. The elevated temperature accelerated the MG's structural changes.
Using a sensitive detector coupled with the intense X-ray beam provided by the APS, the XPCS technique yielded sub-angstrom resolution (dimensions less than one-tenth of a nanometer). Overall, the XPCS measurements, gathered every 2.5 seconds, spanned some 300,000 seconds, or 83 hours. In comparison, similar XPCS experiments with metallic glasses have lasted no more than 17 hours.
The experimental results revealed a decidedly complex interplay of alternating structural changes. For example, periods of decreasing structural change, called monotonic aging, were observed from zero-to-7 hours, 36-to-40 hours, and finally 62-to-70 hours. By contrast, periods of increasing structural change (known as stationary dynamics) occurred around 16½-to-21 hours, 26-to-30½ hours, and finally from 41½-to-46 hours.
Although structural realignments in the metallic glass fluctuated (changed at a faster or slower pace) over most of the experiment, eventually an averaging over the different structural modes described above was observed. This end-stage averaging took the form of non-normal diffusive material transport within the metallic glass. In other words, the rearrangements (diffusions) of atoms within the metallic glass converged towards a single, non-classical dynamic form.
The results from this long-duration experiment considerably expands knowledge of how metallic glasses evolve over time. Ultimately these new insights will help materials scientists develop more stable and higher-performing MGs, which is increasingly important as the demand for these exceptional materials steadily grows. Additionally, the researchers envision development of improved detectors that will reveal new details about the fastest atomic-scale migrations. – Philip Koth
See: B. Riechers1, A. Das2,3, E. Dufresne4, P.M. Derlet5, R. Maaß1,2,6, “Intermittent cluster dynamics and temporal fractional diffusion in a bulk metallic glass,” Nat Commun 15 6595 (2024)
Author affiliations: 1Federal Institute of Materials Research and Testing; 2University of Illinois Urbana-Champaign; 3Cornell University; 4Argonne National Laboratory; 5Paul Scherrer Institute; 6Technical University of Munich
B.R. gratefully acknowledges financial support by the German Aerospace Center (Grant No. 50WM2158), the European Union’s Horizon Europe Framework Program (HORIZON) under the Marie Skłodowska Curie grant agreement (No. 101063523), and BAM’s Adolf Martens postdoctoral fellowship program. The XPCS experiments were performed at the X-ray Science Division beamline 8ID-E of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive X-ray light source facilities. Each year, the APS provides high-brightness X-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other X-ray light source research facility. APS X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
