Inextricably linked with all the life on land, marine phytoplankton generate oxygen and absorb carbon dioxide as a vital part of the biogeochemical cycles that are crucial to sustaining our planet's complex ecosystem. One of the essential elements of those processes is zinc, which is a vital micronutrient for plankton and other marine life. The workings of the global zinc cycle, involving how it moves from one part of the ecosystem to another and in what forms, have been unclear to scientists.
A team of investigators from Princeton University, the University of Stellenbosch in South Africa, the University of California in Santa Cruz, the University of Chicago, and the Max Planck Institute for Chemistry has shed new light on the intricacies of the zinc cycle through the study of water and sediment samples taken from the Southern Ocean, which plays the largest part in its functioning. Their work was published in Science.
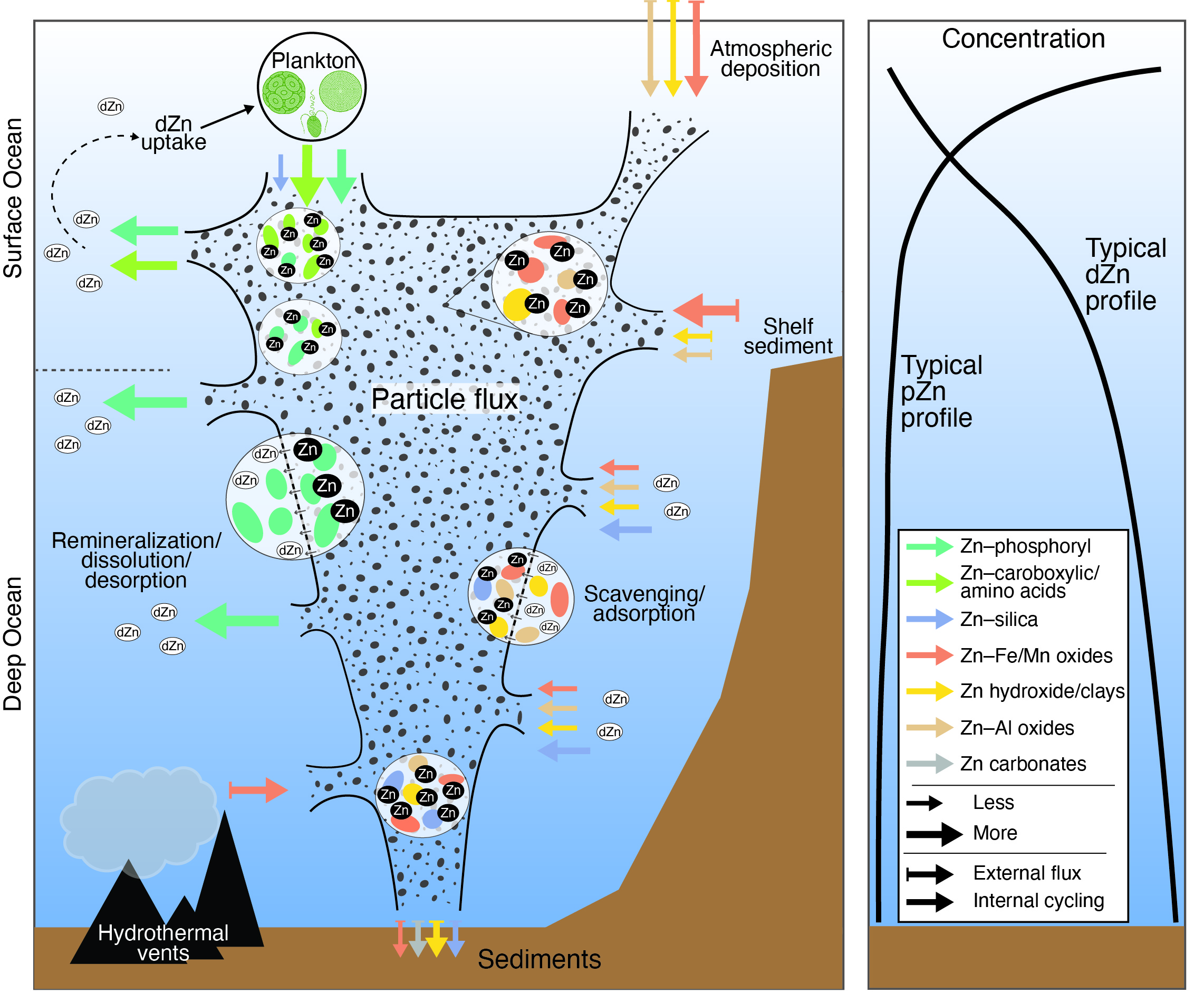
As zinc moves from one part of the ecosystem to another, from the land to sea to living organisms, some zinc (dissolved zinc, or dZn) is dissolved in the photic ocean, while some (particulate zinc, or pZn) binds to different materials including silicates and phosphoryls, which affect its phases, abundance, and availability to biological processes in the various environments. Different species of zinc-containing particles are found in different parts of the Southern Ocean, and can provide clues to how zinc moves from the shallow ocean, where dZn can nourish phytoplankton which generates oxygen and removes carbon dioxide from the atmosphere, to the deep ocean as pZn, where it becomes unavailable to most marine life or is transported to other parts of the world oceans by deep ocean currents and modifies biological productivity beyond the Southern Ocean.
The researchers collected samples from various Southern Ocean water masses in both summer and winter, which were studied by synchrotron X-ray spectroscopy and microscopy to identify the chemical forms of zinc and distribution of other associated elements in the particulate pool. This included X-ray absorption near-edge structure (XANES) spectroscopy and X-ray fluorescence (XRF) imaging at the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Brookhaven National Laboratory, and the University of Chicago’s GeoSoilEnviroCARS beamline at Sector 13 of the Advanced Photon Source, a DOE Office of Science user facility at DOE’s Argonne National Laboratory.
These studies show great variation in the samples depending on the season, location, and depth of sampling. In the summer months, the higher productivity of phytoplankton blooms results in higher levels of biogenic zinc in the surface regions of the ocean, although there is still a considerable amount of lithogenic zinc particles which arise from rock debris, the deposition of atmospheric dust, and the precipitation of inorganic phases. In the winter, however, biogenic zinc levels decline because of poor growing conditions and the ratio between biogenic and lithogenic zinc becomes more balanced. Inorganic solids including silica, as well as iron and aluminum oxides and clay minerals, "scavenge" zinc from biogenic particles and seawater, forming mineral phases which do not easily transform into dZn for easy uptake by plankton.
These particles can form into large aggregates which sink deeper to greater depths, including in sediments, making the zinc they contain unavailable for phytoplankton at shallower depths. As the deep-water masses of the Southern Ocean are sources of surface waters in other parts of the world's oceans, the oceanic conveyor belt can mobilize these nutrients, including zinc, back into surface ocean, thereby promoting plankton growth.
The findings shed new light on some formerly overlooked aspects of the ocean zinc cycle. The lack of large biogenic Zn particle pools and the increase of lithogenic pools in deeper waters of the Southern Ocean have a large effect on phytoplankton productivity, resulting from lower bioavailability of zinc. Because of phytoplankton's crucial role in modulating global carbon dioxide levels, diminished productivity of phytoplankton also has profound implications for the carbon cycle and thus global warming. Although the Southern Ocean plays the largest part in these cycles, similar processes likely occur in other oceans at different latitudes. Further study will yield greater detail on the role and geochemistry of inorganic zinc particles in the global ecosystem. – Mark Wolverton
_____________________________________________________________________________
See: J. Duan1, R. Cloete2, J.C. Loock2, A. Lanzirotti3, M. Newville3, A. Martinez-Garcia4, D.M. Sigman1, P.J. Lam5, A.N. Roychoudhury2, S.C.B. Myneni1, “Biogenic-to-lithogenic handoff of particulate Zn affects the Zn cycle in the Southern Ocean,” Science, 384, 6701, 1235-1240 (June 2024)
Author affiliations: 1Princeton University; 2University of Stellenbosch; 3CARS, University of Chicago; 4Max Planck Institute for Chemistry; 5University of California Santa Cruz.
S.C.B.M. acknowledges the National Science Foundation (CHE; award no. 1609927) and Princeton University (Scott Vertebrate Funds and Phillips Equipment Fund in the Department of Geosciences and student internships in the High Meadows Environmental Institute) for supporting molecular studies. A.N.R acknowledges an anonymous charitable donor trust as part of the Whales and Climate Change Program; the South African National Antarctic Programme (SANAP); the Department of Science and Innovation (DSI); the Department of Forestry, Fisheries and the Environment (DFFE); and the National Research Foundation (NRF) for supporting the sampling and research cruises. This research used resources of the National Synchrotron Light Source II and the Advanced Photon Source, US Department of Energy (DOE), Office of Science User Facilities, operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704 and by Argonne National Laboratory under contract no. DE-AC02-06CH11357, respectively. Portions of this work were performed at GeoSoilEnviroCARS (The University of Chicago, Sector 13) at the Advanced Photon Source. GeoSoilEnviroCARS was supported by the National Science Foundation, Division of Earth Sciences (EAR) (1634415).
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
