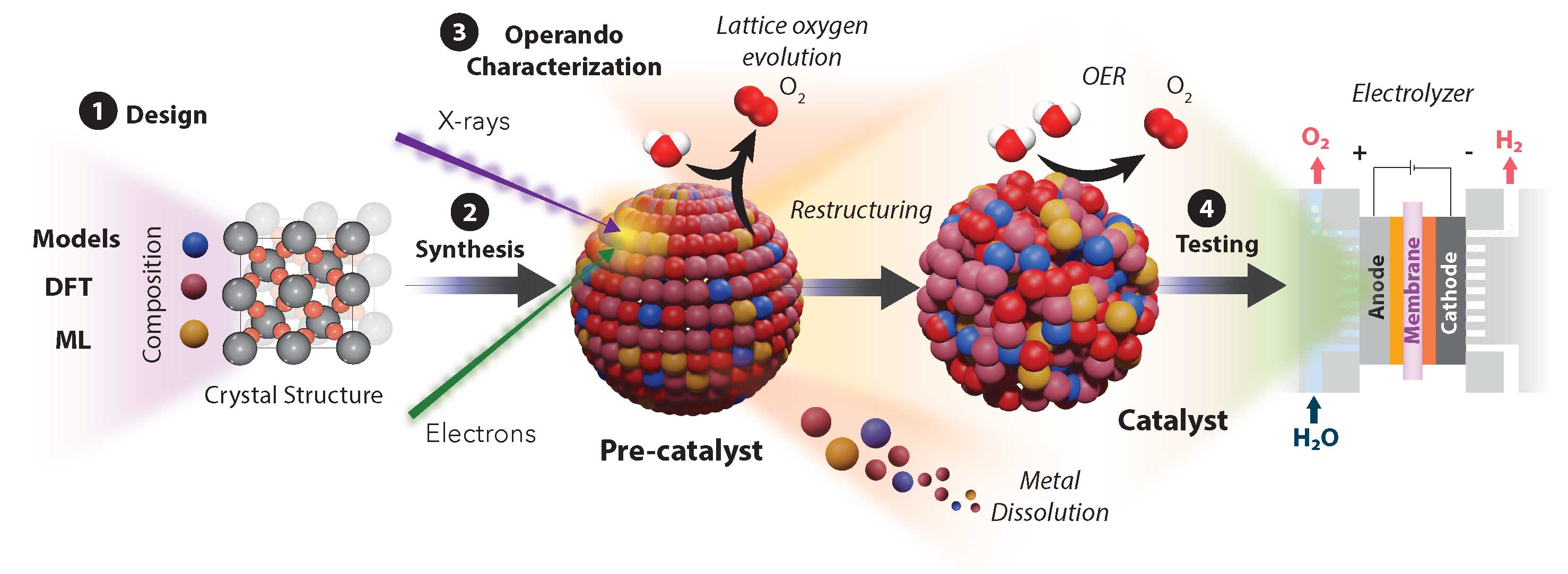
Hydrogen may be the most common element in the universe, but that doesn't mean it's easy to get when we need it, such as for use as an energy source and storage method. "Green hydrogen," as it's known, is generated by splitting water into its component atoms through electrolysis, but that requires materials for an electrolyzer that can catalyze the reaction, some of which are rare and expensive.
Finding alternative electrocatalysts is therefore an important goal in the quest for a carbon-neutral energy grid. But it's a big job because so many chemical possibilities must be evaluated. Researchers from the University of Toronto and Carnegie Mellon University turned to the artificial intelligence technique of machine learning to efficiently screen thousands of possible catalysts and identify some likely choices. Their work appeared in the Journal of the American Chemical Society.
While most commercial electrolysis uses alkaline water electrolyzers, a promising alternative is the proton exchange membrane (PEM) electrolyzer, which uses a solid polymer electrolyte membrane to separate out hydrogen gas at higher pressures and current density than is possible with alkaline electrolyzers. At present, however, the only oxygen evolution reaction (OER) catalyst that can endure the extreme acidic environment at the anode in the PEM electrolyzer is iridium oxide (IrO2), which is expensive because of its great demand for many other uses. In the current work, the researchers explored the prospects for an OER catalyst based on ruthenium in the form of RuO2, which would be a far less expensive and more abundant alternative.
A disadvantage of ruthenium when used in the OER process is its tendency to become overoxidized, with the formation of soluble Ru atoms that can limit its catalytic lifetime and stability. To overcome this problem, the experimenters sought metallic oxides that could alloy with RuO2 and create a more robust and stable OER catalyst. They used a neural net computational pipeline approach applied to density function theory calculations to efficiently screen a large set of mixed metallic oxides to isolate likely candidates.
After training a neural network algorithm model on 36,465 metal oxide structures, the investigators substituted 46 elements in the oxide structure while keeping the rutile oxide structure intact. This led to a set of 2070 hypothetical candidates, which were then evaluated for their Pourbaix electrochemical stability. The investigators note that Pourbaix stability provides an excellent benchmark for gauging the electrochemical stability of catalysts prior to reaction.
Further calculations narrowed down the candidate set to the Ru-Cr-Ti-Ox group, particularly Ti and Cr, so the research team focused on these for experimental validation. They synthesized materials for testing various dopant amounts in the OER catalyst compounds, including in-situ X-ray absorption spectroscopy (XANES) at the 9-BM and 20-BM beamlines of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory.
Through this process, the Ru0.6Cr0.2Ti0.2Ox structure was identified as the best possibility. Various experiments and observations, including transmission electron microscopy (TEM), STXM spectroptychography and XANES, were conducted to determine overpotentials, electrochemical performance, stability and morphology of the sample catalysts. The work revealed that the substitution of Cr and Ti in the ruthenium oxide lattice greatly enhances its stability and electrochemical activity.
Aside from identifying a potentially useful OER catalyst for use in PEM electrolyzers, the work demonstrates a computational solution that could be quite valuable not only for discovering further OER electrocatalysts but candidate materials for other electrochemical applications. Scanning transmission X-ray microscopy also proved to be extremely valuable for observing spatial and temporal changes over time, and to map metal-oxygen covalency improvements across the nanoparticle.
The researchers believe that future research should focus on optimizing and engineering catalyst deposition on the membrane for high-current PEM operation and also identifying the synthesis technique that yields the most stable and conductive layer on the PEM membrane. – Mark Wolverton
_____________________________________________________________________________
See: J. Abed1, J. Heras-Domingo2, R.Y. Sanspeur2, M. Luo3, W. Alnoush4, D.M. Meira5,6, H. Wang6, J. Wang6, J. Zhou6, D. Zhou1, K. Fatih7, J.R. Kitchin2, D. Higgins4, Z.W. Ulissi2, E.H. Sargent1, “Pourbaix machine learning framework identifies acidic water oxidation catalysts exhibiting suppressed ruthenium dissolution,” J. Am. Chem. Soc., 2024, 146, 23, 15740-15750 (June 2024).
Author affiliations: 1University of Toronto; 2Carnegie Mellon University; 3Peking University; 4McMaster University; 5Argonne National Laboratory; 6Canadian Light Source; 7National Research Council of Canada.
This work was supported financially by the National Research Council (NRC) of Canada, the Natural Sciences and Engineering Research Council (NSERC) of Canada, a Vanier Canada Graduate Scholarship, and the Army Research Office (ARO) project Award W911NF2010188. Electron microscopy, scanning transmission electron microscopy, and electron energy loss spectroscopy were performed at the Canadian Centre for Electron Microscopy (CCEM) at McMaster University. STXM ptychography was performed at the SM beamline at the Canadian Light Source, a national research facility of the University of Saskatchewan, which is supported by the Canada Foundation for Innovation (CFI), NSERC, NRC, the Canadian Institutes of Health Research (CIHR), the Government of Saskatchewan, and the University of Saskatchewan. This research used resources of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. Department of Energy (DOE) Office of Science by Argonne National Laboratory and was supported by the U.S. DOE under contract no. DE-AC02-06CH11357, and the Canadian Light Source and its funding partners. The authors thank Dr. Tianpin Wu and Dr. George Sterbinsky from 9BM beamline, and Debora Motta Meira and Zou Finfrock from 20BM beamline for assistance in collecting the XAS data and at the advanced photo source (APS). This paper is an adaptation of the author’s thesis titled “Developing Efficient Electrocatalysts for Oxygen Evolution at High Current Densities” submitted to University of Toronto.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
