Modern electronic devices rely on semiconductors that incorporate minute amounts of a foreign element. This process of careful contamination is called doping. The semiconducting element silicon, for instance, is often doped with tiny amounts of phosphorus or boron, depending upon the desired electronic properties.
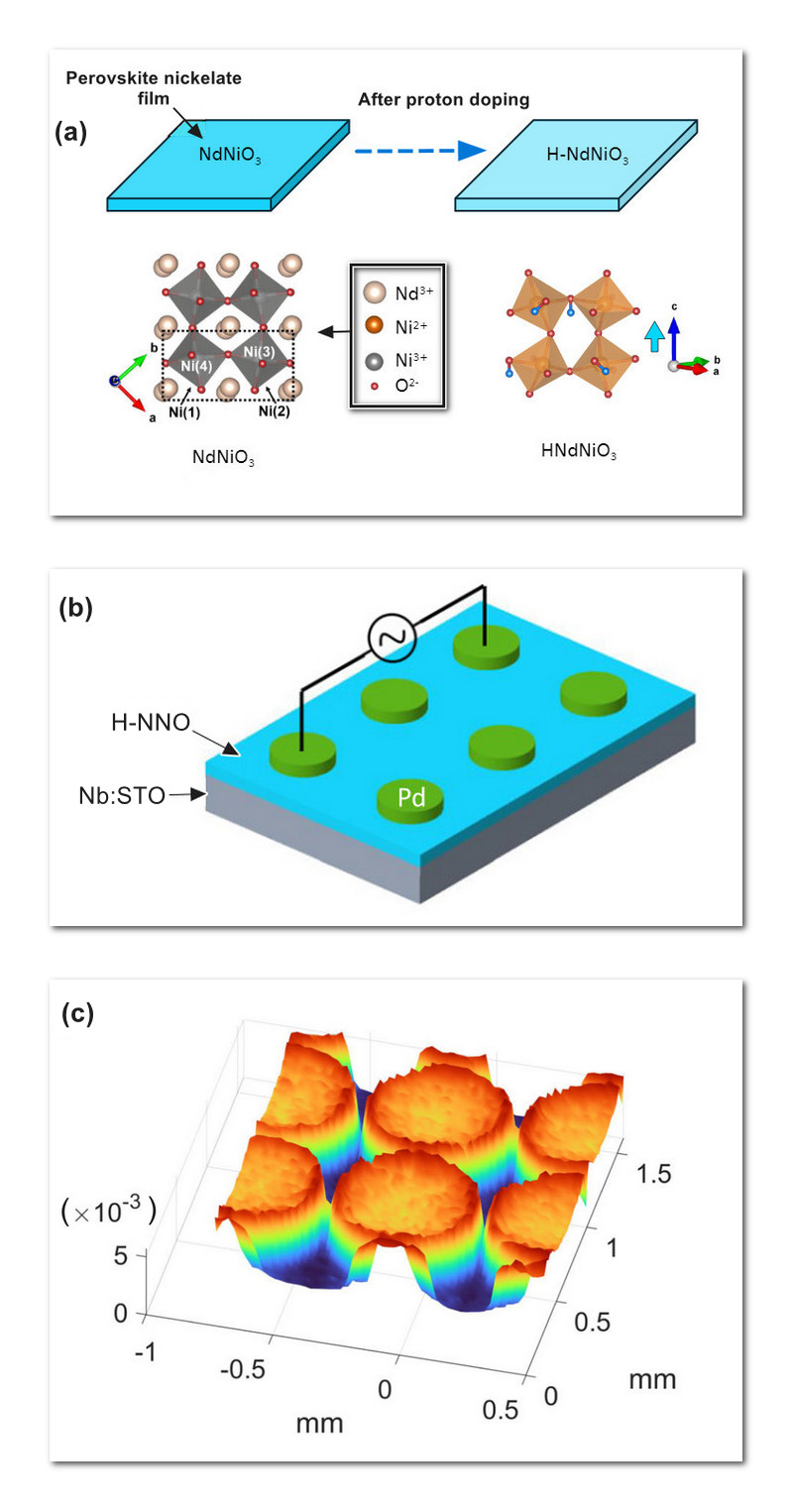
A less familiar type of doping involves migrating hydrogen into a host material. Hydrogen doping, or hydrogenation, has been applied to many materials, including oxide compounds with complex crystalline structures. A representative example is the neodymium-nickel oxide NdNiO3. A broad-based research team recently explored the structure of NdNiO3 post-hydrogenation (denoted H-NNO). Synchrotron X-ray measurements performed at the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, played a vital role in characterizing the hydrogenated compound.
The researchers demonstrated, for the first time, that transient polarization can spontaneously arise in H-NNO. The transient polarization lasted nearly a second before disappearing. Furthermore, the researchers showed that the extent of polarization can be adjusted. The ability to manipulate polarization in H-NNO could find use in a variety of applications, such as powder-coating and photocopying technique xerography. The scientists also performed tantalizing experiments indicating the potential of H-NNO for neuromorphic computing, which mimics human brain processes.
Electrical polarization can occur when charged particles in a material's molecules slightly shift in response to an external electric field. A flat-plat capacitor embodies this externally induced polarization: two closely spaced flat metal plates are separated by an insulating material such as plastic film. When connected to a voltage source, one plate becomes positively charged and the other negative. Due to the electric field produced by the oppositely charged plates, the molecules in the insulator are stretched, with their negative electrons pulled towards the positive plate and positive ions towards the negative plate.
Spontaneous polarization, by contrast, arises in ferroelectric materials without any applied voltage. Ferroelectric compounds are used as variable capacitors and in other electronic applications. While scientists knew that thin-film NdNiO3 turns from electrically conducting into a nonconducting insulator following hydrogenation, it was unclear if hydrogenation also produces spontaneous polarization.
For this study a thin film of NdNiO3 was probed at room temperature. NdNiO3 is part of the perovskite family of crystalline compounds that have been extensively studied for their unusual electric and magnetic properties. Perovskites are composed of octahedral crystalline units. The researchers crafted a thin film of NdNiO3, approximately 0.15 micrometers thick, with palladium electrodes attached. The palladium accelerated diffusion of hydrogen into the film. A variety of observational techniques, such as optical imaging, were used to characterize the H-NNO film. At the APS, synchrotron X-ray reflexivity and X-ray microdiffraction measurements were performed at beamline 12-ID-D.
Hydrogen atoms possess a single proton. As hydrogen disperses into the NdNiO3 crystalline lattice, their protons are pulled into lattice voids while their electrons become localized to the nickel-oxide octahedra. X-ray data revealed an uneven distribution of donated protons within the H-NNO film, with higher concentrations near the palladium electrodes.
Theoretical calculations based on the X-ray results indicated the presence of both polar capacitance, meaning charge separation within individual molecules (like in a flat-plate capacitor), accompanied by space-charge capacitance encompassing larger regions. The space-charge capacitance, resulting from excessive positive charge due to the captured protons, dominated when an applied voltage reached a threshold value. The experimental results also demonstrated that the film's spontaneous polarization can be tuned by an appropriate voltage.
Because the donated protons can migrate between voids, spontaneous capacitance in the H-NNO film is transient, subsiding within a second. This is actually a long duration compared to many electronic circuits that cycle billions of times per second. The researchers used this relatively long-lived transient capacitance to simulate neural networks designed to recognize the digits 1 through 9 and achieved a high rate of image recognition (around 90% accuracy).
This study demonstrates spontaneous polarization in room-temperature hydrogenated NdNiO3 films, which can be tuned by adjusting the amount of hydrogen doping. The transient nature of the polarization makes it promising for neural network applications. Moreover, the use of hydrogen doping to discover spontaneous capacitance should be applicable to many other thin-film materials, including multiferroic compounds that exhibit both electric and magnetic properties of interest. Hydrogen doping is therefore a novel process to manipulate properties of quantum materials for use in emerging microelectronics. – Philip Koth
_____________________________________________________________________________
See: Y. Yuan1, M. Kotiuga2, T.J. Park3, R.K. Patel1, Y. Ni4, A. Saha5, H. Zhou6, J.T. Sadowski7, A. Al-Mahboob7, H. Yu3, K. Du1, M. Zhu1, S. Deng3, R.S. Bisht1, X. Lyu3, C-T. M. Wu1, P.D. Ye3, A. Sengupta5, S-W. Cheong1, X. Xu4, K.M. Rabe1, S. Ramanathan1, “Hydrogen-induced tunable remanent polarization in a perovskite nickelate,” Nat Commun., 15, 4717 (June 2024).
Author affiliations: 1Rutgers, State University of New Jersey; 2Ecole Polytechnique Federale de Lausanne; 3Purdue University; 4University of Nebraska Lincoln; 5Pennsylvania State University; 6Argonne National Laboratory; 7Brookhaven National Laboratory.
This research used resources of the Center for Functional Nanomaterials and the National Synchrotron Light Source II, which are U.S. Department of Energy (DOE) Office of Science facilities at Brookhaven National Laboratory, under Contract No. DE-SC0012704. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. The authors acknowledge AFOSR grant FA9550-22-1-0344 for supporting the compositional characterization and polarization studies of the films. K.D. and S.W.C. were supported by the DOE under Grant No. DOE: DE-FG02-07ER46382. We acknowledge the Laboratory for Surface Modification at Rutgers University for ERDA measurements.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
