A new class of medicines called PROTACs (PROteolysis TArgeting Chimera) represents an entirely new mode of action for small molecule drugs. They destroy disease-causing proteins by hijacking the cell’s degradation machinery—the ubiquitin proteasome pathway (UPP). Now, a multidisciplinary team of structural biologists, cell biologists, and chemists has engineered a PROTAC molecule that degrades two proteins that promote cancer, without causing off-target side effects.
The scientists used data collected at the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, as the basis for structural studies that paved the way for the PROTAC’s development. Their research was published in the journal Nature Communications and describes how solving the crystal structures of the PROTAC in complex with its targets and E3 ligase (termed as ternary complex) holds the promise of a potent new way to fight cancer and other diseases.
Rather than interfering with or inhibiting proteins of interest, like existing small molecule drugs do, PROTACs degrade proteins by co-opting one of the main metabolic pathways that target proteins for destruction. Moreover, unlike traditional small molecule drugs, which need to find a specific binding pocket on the target protein, PROTACs just need to recognize the protein and attach anywhere on its surface.
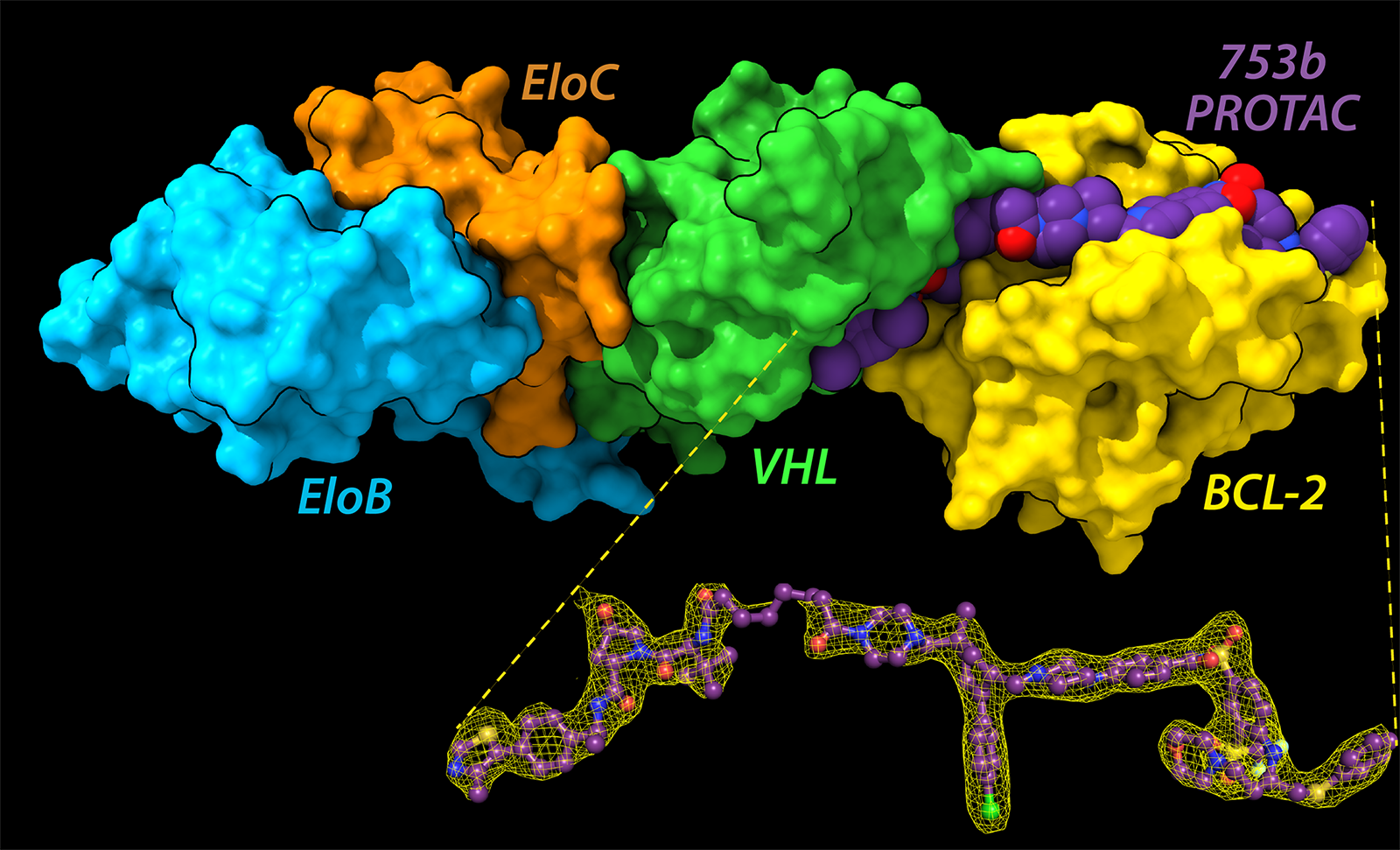
A PROTAC molecule comprises three subunits in the shape of a barbell: a subunit that attaches to the protein of interest; a subunit that attaches to an enzyme called E3 ligase, which tags a protein with ubiquitin (a sort of “destroy me” flag); and a linker that connects the two subunits. The PROTAC brings the target protein and the E3 ligase in proximity so that the enzyme can tag the target protein with ubiquitin and mark it for destruction.
The interactions created by PROTACs do not occur in nature. PROTACs target proteins that would normally (in the absence of PROTACs) never see the E3 ligase, fostering new atomic connections. These de novo contacts, as they’re called in the PROTAC field, can affect the behavior—hence the potency—of the PROTAC, depending on how many connections there are and whether they can hold the ternary complex in a stable configuration.
In the current work, the target proteins are BCL-xL and BCL-2, both of which enable cells to avoid programmed cell death (apoptosis) and are highly expressed in cancer cells. The team had previously designed a PROTAC called DT2216, currently under clinical investigation. However, it only targets BCL-xL in the cell, and the team wanted to target BCL-2 also.
Through rational design guided by computer modeling, they engineered a new PROTAC, 753b, based on DT2216. They then wanted to harness structural biology to study the mode of action of 753b and make it more effective in the cell. That meant investigating how 753b worked on an atomic level.
The team developed an innovative method for purifying out a homogenous ternary complex of E3/753b/BCL-xL and E3/753b/BCL-2, respectively which was crucial for crystal formation. However, these crystals were tiny—too tiny for the team to resolve at their home lab. They sent the crystals to the APS, where they collected a complete data set at the Northeastern Collaborative Action Team (NE-CAT) beamline 24-ID. Hence the team was able to solve two ternary complexes at atomic resolution.
The crystal structures showed that the atomic interactions at the interface between the linker and the target protein differed in the two ternary complexes, creating two different architectures: 753b was linear in case of BCL-2 complex, while for BCL-xL complex, 753b took an L-shape.
The team hypothesized that replacing an uncharged portion of the linker with a polar molecule would enable the PROTAC to make more electrostatic contacts with the target protein, stabilizing the ternary complex overall. They could also see that the length of the linker was perfect, and that interfering with it could hamper the ternary complex formation. Thus, they incorporated a charged molecule in the linker, keeping the linker length same as that of 753b. Moreover, the team also made some changes on the PROTAC based on rational design.
Voila, a new PROTAC was born: WH244. The team made ternary complexes with WH244 similar to those they’d made with 753b, then solved the crystal structures at the NE-CAT beamline. The team tested its potency in human cancer cells and tested it for off-target side effects using a proteomics assay. Out of 7200 proteins detected, the new PROTAC degraded only its two intended targets both quickly and effectively with a small dose of drug. Moreover, it improves on DT2216 by degrading BCL-2 in addition to BCL-xL in cells.
The authors’ goal is to apply the knowledge they’ve gained from their structural studies to the development of medicines that can eradicate cancer. Their work also has a broader impact, in that the method they developed to isolate a homogenous ternary complex in the first place can be applied to other ternary complexes with different target proteins.
This multidisciplinary team has been working together for a long time. Their next step is to identify additional E3 ligases present in other cell types so their PROTACs can be used in many different tissue types. To date, no PROTAC has received FDA approval, but once that happens, interest and investment in this new area of drug development are projected to skyrocket. – Judy Myers
See: D. Nayak1, D. Lv1, Y. Yuan1, P. Zhang2, W. Hu2, A. Nayak1, E.A. Ruben1, Z. Lv1, P. Sung1, R. Hromas1, G. Zheng2, D. Zhou1, S.K. Olsen1, “Development and crystal structures of a potent second-generation dual degrader of BCL-2 and BCL-xL,” Nature Communications, 15, 2743 (March 2024)
Author affiliations: 1University of Texas Health Science Center; 2University of Florida
The authors thank members of the Zheng, Zhou, and Olsen laboratories for helpful discussions. Research reported in this publication was supported by NIH grants R01 GM115568 and R01 GM128731 and CPRIT Rising Star Award RR200030 (S.K.O.). R01 CA242003, R01 CA241191, and R01 AG063801 (G.Z. and D.Z.). NCI R21 CA286307 and a Mays Cancer Center Early Career Pilot Award from CCSG (NIH P30 CA054174) (D.L.). The X-ray diffraction data were collected on beamline NE-CAT 24-ID-E at the Advanced Photon Source, Argonne National Laboratory. The DIA Mass spectrometry analyses were conducted at the University of Texas Health Science Center at San Antonio Institutional Mass Spectrometry Laboratory, with expert technical assistance of Sammy Pardo and Dana Molleur, supported in part by NIH grant P30 CA54174-23 (S.T. Weintraub, Mays Cancer Center Mass Spectrometry Shared Resource) and NIH grant S10 OD030371-01A1 (S.T. Weintraub) for purchase of the Orbitrap Exploris 480 mass spectrometer. This work is based upon research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by the National Institute of General Medical Sciences from the National Institutes of Health (P30 GM124165). The Eiger 16M detector on 24-ID-E is funded by an NIH-ORIP HEI grant (S10OD021527). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. This research utilized resources of the Structural Biology Core Facilities, part of the Institutional Research Cores at the University of Texas Health Science Center at San Antonio supported by the Office of the Vice President for Research and the Mays Cancer Center Drug Discovery and Structural Biology Shared Resource (NIH P30 CA054174) and the Center for Innovative Drug Discovery (CPRIT Core Facility Award RP210208). The Rigaku HyPix-6000HE Detector, Universal Goniometer, and VariMax-VHF Optic instrumentation in the Structural Biology Core Facilities are funded by NIH-ORIP SIG Grant S10OD030374. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive X-ray light source facilities. Each year, the APS provides high-brightness X-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other X-ray light source research facility. APS X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
