The lanthanides and other rare earth elements (REEs) aren't really "rare" in the strict sense, but they are quite difficult to separate and purify from the other materials with which they're usually found. Because of the great value and utility of these metals for many purposes, including electronics, computing, and various industrial processes that rely on their unique electronic and chemical properties, that difficulty is a major problem.
Most current processes for REE separation and purification involve organic and acidic materials, making them both energy-intensive and environmentally unfriendly. Finding better separation techniques is therefore a pressing challenge. Researchers from the University of Chicago, Northwestern University, and Argonne National Laboratory took inspiration from nature to examine a new possibility for lanthanide separation. Their work was published in Science Advances.
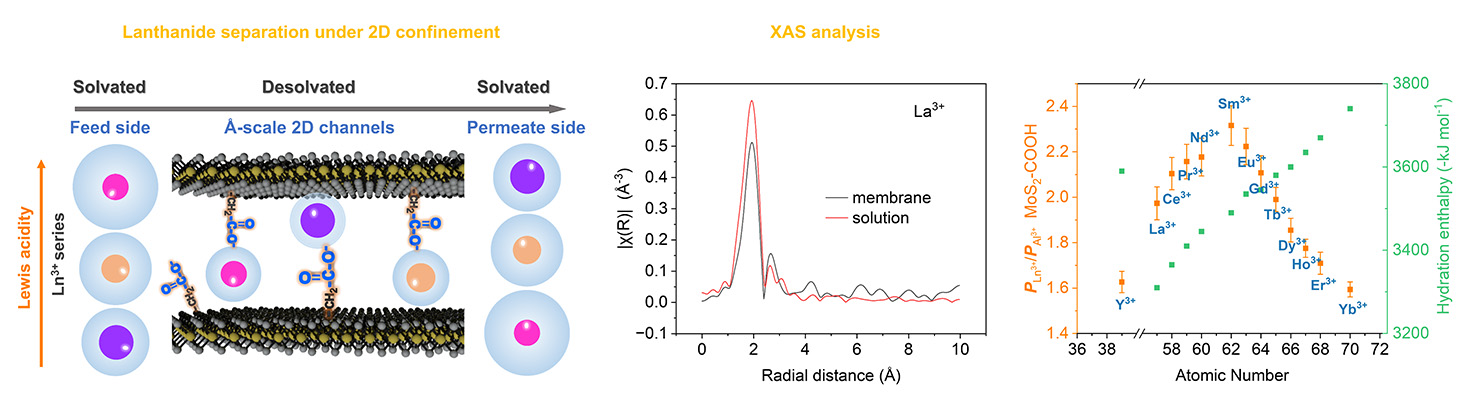
Noting that ion channels in cell membranes are capable of separating ions across cell membranes with great efficiency, speed and selectivity, the investigators chose to model this process with chemically functionalized inorganic membranes to see if REE purification could be accomplished in a similar way. They constructed two-dimensional angstrom-scale artificial ion channels using MoS2 nanosheets that were covalently functionalized with acetic acid to generate MoS2-COOH membranes for lanthanide ion separation.
The ion transport process was studied using a variety of tools, including electron microscopy, infrared spectroscopy, molecular dynamics simulations and X-ray absorption spectroscopy and X-ray diffraction studies. Data were collected at the DuPont-Northwestern-Dow Collaborative Access Team 5-BM-D beamline at the Advanced Photon Source, a U.S. Department of Energy (DOE) user facility at DOE’s Argonne National Laboratory.
The experimental model consisted of a mixture of 13 REE cations of different species in the feed and an identical volume of deionized water initially in the permeate, with stacked MoS2-COOH two-dimensional angstrom-scale nanosheets as the membrane in between, using osmotic pressure as the driving force. The average height of the ion transport channels was shown to be approximately 6.3 angstroms.
Plots of the ion transport trends through the angstrom-scale channels show a clear volcano shape which peaks at Sm3+, decidedly unusual for lanthanides due to the properties caused by the lanthanide contraction (shrinkage of the ions as one progresses to heavier lanthanides). This volcano shape persists even with the use of different ion mixtures. The researchers observed in additional tests using a polytetrafluoroethylene (PTFE) membrane for comparison that the confinement in the extremely narrow 2D channel of MoS2-COOH improves the separation selectivity of REE ions.
The researchers investigated this possibility further by detailed examination of the 2D REE thermodynamics and kinetics, including ion uptake, the interaction of lanthanide ions with the confinement channels, and the effect of the dehydration barrier. Allowing for the simplified model used in the MD simulations, the findings are remarkably consistent with the experimental results.
The work offers some interesting prospects for developing greener techniques for REE separation and purification. By tuning selectivity in 2D channels using different functional groups, controlling dehydration, and otherwise adjusting the chemistry and confinement dimensions, very efficient separation capabilities can be achieved. Ideally, this could lead to greatly reduced use of the environmentally damaging components upon which current REE separation techniques depend, while also making more REE materials readily and less expensively available for all their critical uses in the 21st century, including the vital effort to reduce carbon usage. – Mark Wolverton
_____________________________________________________________________________
See: M. Wang1, Q. Xiong2, M. Wang3, N.H.C. Lewis1, D. Ying1, G. Yan1, E. Hoenig1, Y. Han1, O-S. Lee2, G. Peng1, H. Zhou3, G.C. Schatz2, C. Liu1, “Lanthanide transport in angstrom-scale MoS2-based two-dimensional channels,” Sci Adv 10, eadh1330 (March 2024)
Author affiliations: 1University of Chicago; 2Northwestern University; 3Argonne National Laboratory;
This work is supported by the Department of Energy, Office of Basic Energy Science, under grant DE-SC0022231. This work made use of instruments in the Electron Microscopy Core of UIC’s Research Resources Center. This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. Portions of this work were performed at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) located at Sector 5 of the APS. DND-CAT is supported by Northwestern University, The Dow Chemical Company, and DuPont de Nemours, Inc.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
