Scientists are always searching for new catalysts to enable fast, energy-efficient chemical reactions to transform wastes into useful chemical fuels, such as converting carbon dioxide to methane. Single-site catalysts are a promising new class of catalyst, with well-defined, well-designed structures where each reaction site is isolated. The challenge with such materials is that the active catalytic sites, which can be as small as a single atom, tend to aggregate, degrading their efficiency and selectivity.
Now researchers using the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, have demonstrated a design for such single-site catalysts that resist aggregating and retain their high efficiency.
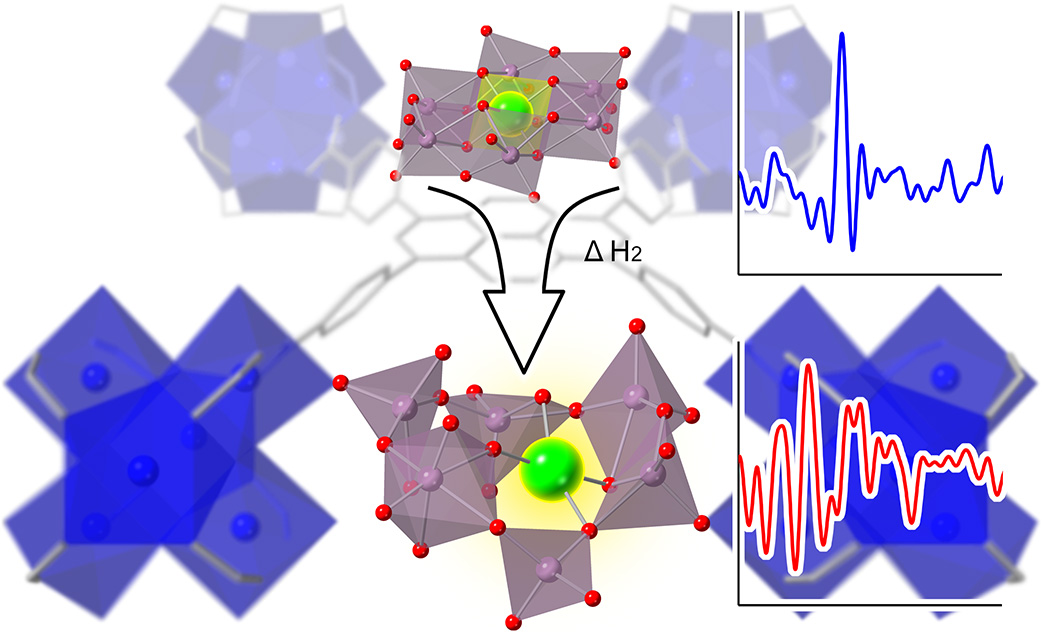
The design starts with polyoxometalate clusters (POMs). These are discrete polyatomic anions in which three or more metals, including molybdenum, share oxygen atoms. Catalytic atoms, often noble metals such as platinum or rhodium, can be embedded with the POM. The POM essentially belts the single-site catalyst atom in place, so it has difficulty interacting with other catalytic sites.
But for the catalyst to be efficient, reacting molecules need to be able reach the active site, so the next step is to disperse them through a support with high surface area. In this case, the researchers used a zirconium-based metal-organic framework (MOF), a porous architecture that contains a high surface area within a small volume. This dual-confinement strategy allows the researchers to achieve a relatively high density of active sites, up to 3.2 weight percent, without the sites sintering together during use.
One previous strategy to prevent aggregation of the catalytic atoms had been to spread them far apart on a support material. That made it difficult to assess the structure of the catalysts. The high catalyst loading that is stable using the POM-MOF approach provides sufficient signal to see the catalyst sites using X-rays. The team used pair distribution function analysis (PDF) a technique that is a specialty of beamline 11-ID-B at the APS. PDF provides the structure and relative geometry of the atoms that make up the active catalyst site. Researchers heated samples of their material to 200 °C and used scattering measurements to determine the structure of the POMs when they entered an active state.
The team also performed X-ray absorption spectroscopy (XAS) at beamline 5-BM-D, the Dupont-Northwestern-Dow Collaborative Access Team (DND-CAT) beamline. This technique provides element-specific information, allowing them to isolate the platinum and the rhodium environments. Combining the PDF and XAS measurements allowed the researchers to figure out the local geometry of the catalyst’s active site during the catalytic reaction. They found, for instance, that the distance between the rhodium or platinum and the molybdenum in the active catalyst was shorter than the metal-to-metal bond lengths in bulk metals.
Researchers were able to compare their experimental data with computer simulations and say which of almost 100 computer-generated models most closely matched their results. Now that they’ve identified the structure of their single-site catalysts and refined the models, they can undertake further computational studies to explore what other formulations might be most promising, before trying to synthesize them.
Armed with this new understanding of POMs, scientists can now explore whether there are different versions that might achieve high efficiency while using cheaper or more abundant metals than platinum, making chemical reactions more energy and cost efficient. – Neil Savage
See: Z. Chen1, S.M.G. Rabbani2, Q. Liu3,4, W. Bi3,4, J. Duan3, Z. Lu3, N.M. Schweitzer3, R.B. Getman2, J.T. Hupp2, K.W. Chapman1, “Atomically precise single-site catalysts via exsolution in a polyoxometalate-metal-organic framework architecture,” Journal of the American Chemical Society 2024, 146, 12, 7950-7955 (March 2024)
Author affiliations: 1Stony Brook University; 2Clemson University; 3Northwestern University; 4University of Science and Technology of China
This work was initially supported as part of the Inorganometallic Catalyst Design Center, an Energy Frontier Research Center (EFRC) funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences (DE-SC0012702), and subsequently as part of the Catalyst Design for Decarbonization Center EFRC (DE-SC0023383). Q.L. and W.B. acknowledge the financial support as visiting scholars from the National Natural Science Foundation of China (No. 11705205, No. 22175051). This research used the beamline 5-BM-D for X-ray adsorption spectroscopy and 11-ID-B for total scattering measurements at the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. This research used ambient pressure XPS of the Center for Functional Nanomaterials (CFN), which is a U.S. Department of Energy Office of Science User Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. This work made use of the Reactor Engineering and Catalyst Testing (REACT) core facility of the Center for Catalysis and Surface Science at Northwestern University. The authors acknowledge Leighanne C. Gallington and Qing Ma for the help with remote measurements at the beamline. We thank Ashley R. Head for help with setting up the APXPS measurement. Q.L. thanks Yang Song and Wangsheng Chu for the analysis of XAS.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive X-ray light source facilities. Each year, the APS provides high-brightness X-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other X-ray light source research facility. APS X-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
