Ever had your phone heat up in your hand? Scientists are exploring materials whose electronic properties could reduce the amount of resistive heating created by the transistors in computer chips. These new materials incorporate a one-dimensional electronic structure---meaning that the electrons which create current are confined to a single dimension---inside a three-dimensional crystal with insulating properties.
It isn't straightforward, however, to synthesize a material with true one-dimensional conductive components and no electronic interactions in the other dimensions. Recent research results by a team whose members collaborate from universities in Spain, Germany and the United States propose and evaluate the chemical criteria required to synthesize such a material and demonstrate the ability of a bulk crystal (BiIr4Se8) fabricated according to such criteria to produce one-dimensional electronic structure.
The team reasoned that such a bulk crystal would need to have the following three characteristics. First, the material would need to include a one-dimensional conductive component and an insulating component which physically surrounds the conductive one. Second, the atoms of the conductive component would need to have charged particles available in their outermost electron shell so those particles could move and produce a current. Lastly, the insulating and conductive components must have no electronic interactions; this is most easily accomplished by choosing components which do not form covalent bonds.
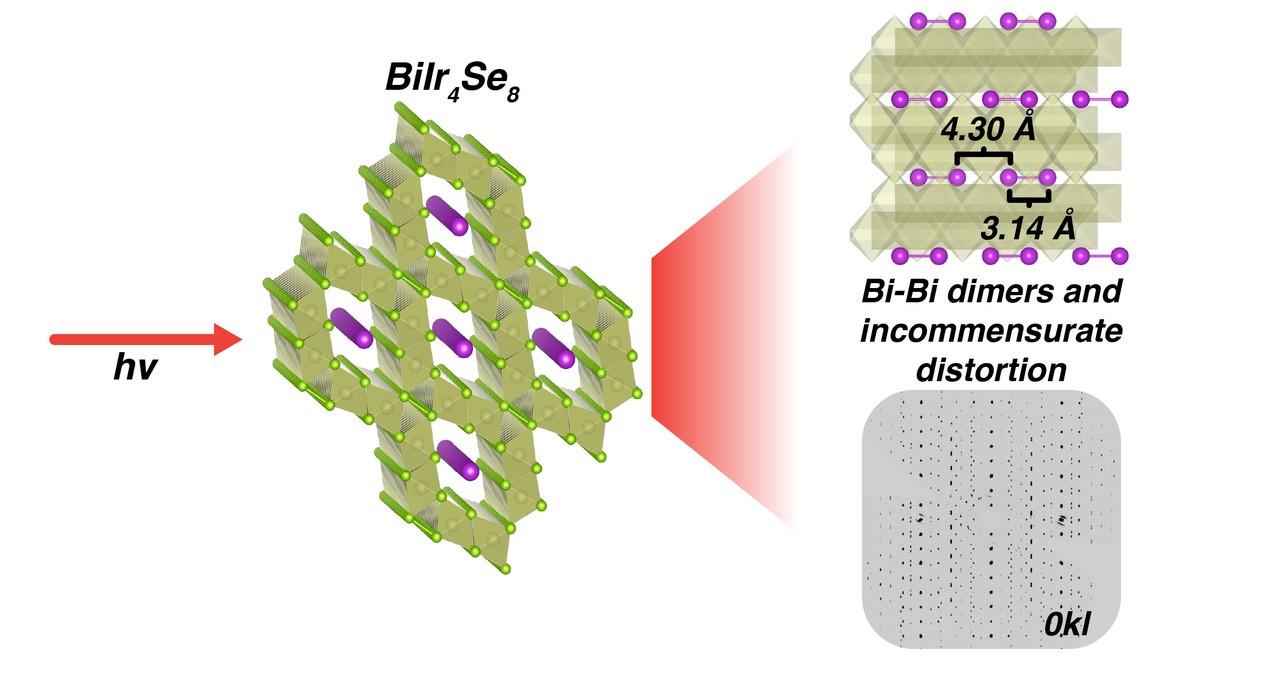
Previously published literature on this topic explored a crystal structure (called "hollandite") that consists of octahedral tunnels formed by one compound (termed the scaffolding) surrounding a second compound whose shape is linear (called the chain). The team decided to use a variant of the hollandite structure that consists of scaffolding made from iridium hexaselenium (IrSe6) and chains of bismuth atoms; the bulk crystal structure is BiIr4Se8.
To characterize the physical and electronic structure of the BiIr4Se8 bulk crystal, the team performed multiple types of examination. They used previously published high-symmetry structures to perform electron counting, which assigns valence electrons to the atoms which make up the crystal's unit cell; this exercise told them the bismuth atoms have an unpaired single electron in their outer orbital, which would require stabilization. Stabilization would most likely occur through bonding between bismuth atoms. The team applied density functional theory---which predicts a material's electronic behavior based on quantum mechanics---to calculate that the crystal would contain a conductive component (a metallic band) and that component was concentrated around the bismuth chain.
Additionally, the team calculated the phonon dispersion relation, which showed two important results. First, it implied the chain and the scaffolding are largely electronically decoupled. Second, it confirmed the electron counting results: a bismuth chain of equally spaced atoms is electronically unstable, implying that the bismuth atoms in the chain should clump together in pairs. This bonding is called dimerization. Figure 1 depicts the scaffolding and chain structure of the bulk crystal, as well as the experimental evidence showing the dimerized bismuth atoms.
This clumping of bismuth atoms is the most vital characteristic that indicates one-dimensional electronic behavior is occurring. The clumping implies that chemical interactions are primarily within the bismuth chain and not in other directions. Since the electronic characterization supports the presence of this clumping but bulk crystal measurements were inconclusive, the team assessed a single BiIr4Se8 crystal for experimental evidence of a bismuth-bismuth bond: doubling of the crystal's unit cell in the direction of the bismuth chain.
The team used the University of Chicago’s ChemMatCARS beamline at 15-ID at the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, to collect synchrotron X-ray diffraction data to ascertain that doubling was occurring. The team further investigated the crystal at low temperatures. Based on the additional measurements, the team found that another bonding distortion was also present in the bismuth chain. Thus, while a dimerization is present, additional bonding more complex than dimerization is occurring within the bismuth chain.
The team's results show that BiIr4Se8 has potential for applications requiring one-dimensional electronic behavior from three-dimensional bulk crystals, ranging from exotic applications such as quantum computing to simply cooling off the phone in your pocket. – Mary Agner
_____________________________________________________________________________
See: C.J. Pollak1, G. Skorupskii1, M. Gutierrez-Amigo2,3,4, R. Singha1, J.W. Stiles1, F. Kamm1, F. Pienhofer5, N.P. Ong1, I. Errea2,3,4, M.G. Vergniory4,6, L.M. Schoop1, “Chemical bonding induces one-dimensional physics in bulk crystal BiIr4Se8,” J. Am. Chem. Soc. 2024, 146, 10, 6784-6795 (March 2024)
Author affiliations: 1Princeton University; 2University of the Basque Country; 3Centro de Fisica de Materiales; 4Donostia International Physics Center; 5University of Regensberg; 6Max Planck Institute for Chemical Physics of Solids.
This research was primarily supported by the Princeton Center for Complex Materials, a National Science Foundation (NSF)-MRSEC program (DMR-2011750), the Gordon and Betty Moore Foundation’s EPIQS initiative (grant numbers GBMF9064 and GBMF9466), and the David and Lucille Packard foundation. C.J.P. is supported by the NSF Graduate Research Fellowship Program under grant number DGE-2039656. G.S. is supported by the Arnold and Mabel Beckman foundation through an AOB postdoctoral fellowship. NSF’s ChemMatCARS, Sector 15 at the Advanced Photon Source, Argonne National Laboratory, is supported by the Divisions of Chemistry (CHE) and Materials Research (DMR), National Science Foundation, under grant number NSF/CHE-1834750. This research used resources of the Advanced Photon Source, a U.S. Department of Energy Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under Contract no. DE-AC02-06CH11357.M.G.V., I.E., and M.G.A acknowledge the Spanish Ministerio de Ciencia e Innovacion (grants PID2019-109905GB-C21, PID2022-142008NB-I00, and PID2022-142861NA-I00). I.E. acknowledges the Department of Education, Universities and Research of the Eusko Jaurlaritza and the University of the Basque Country UPV/EHU (Grant no. IT1527-22). M.G.V. thanks support to the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) GA 3314/1-1─FOR 5249 (QUAST) and partial support from European Research Council grant agreement no. 101020833. M.G.A. thanks the Department of Education of the Basque Government for a predoctoral fellowship (grant no. PRE_2019_1_0304). This work has been financially supported by the Ministry for Digital Transformation and of Civil Service of the Spanish Government through the QUANTUM ENIA project call - Quantum Spain project, and by the European Union through the Recovery, Transformation and Resilience Plan - NextGenerationEU within the framework of the Digital Spain 2026 Agenda. C.J.P. acknowledges Scott Lee and Jason Khoury for useful discussions.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
