The anode in conventional lithium-ion batteries (LIBs) is made of graphite, a layered material that allows lithium ions to reversibly slide between layers during rechargeable battery cycling. The success of these batteries relies on a solid-electrolyte interphase (SEI) layer on graphite. Formed from electrolyte reduction on the first cycle of a LIB’s use, this thin, homogenous layer protects the electrolyte from further degradation.
The solvents used in electrolytes in earlier iterations of LIBs, such as propylene carbonate (PC), tetrahydrofuran (THF), or 1,2-dimethoxyethane (DME), have been found to cointercalate in graphite along with the lithium ions due to limited SEI formation. This cointercalation process produces ternary graphite-intercalation compounds (t-GICs), which can be structurally stable depending on the solvents used. Compared to PC, ether-based solvents have the potential for reversible cointercalation and excellent reductive stability and have been explored extensively in sodium-ion and potassium-ion batteries.
However, some studies have suggested that the t-GICs from ether-based solvents are unstable in LIBs, causing exfoliation of graphite and battery failure at large current densities. Using a specially designed electrolyte, a team of researchers from Virginia Tech, the U.S. Department of Energy’s (DOE) Argonne National Laboratory, Gachon University, Boise State University, and DOE’s SLAC National Accelerator Laboratory showed that it’s possible to achieve long-cycling graphite anode LIBs with an ether-based solvent. The research team used beamlines 17-BM and 12-ID-D of the Advanced Photon Source (APS), a DOE Office of Science user facility at Argonne. Their findings could lead to LIBs with high energy retention able to operate under extreme conditions.
The researchers formulated the new electrolyte by combining DME with 1M LiBF4. They purposely chose LiBF4 as the electrolyte’s salt for qualities compatible with the cointercalation mechanism, including its ability to limit SEI formation and its electrocompatibility with lithium.
Cyclic voltammetry measurements in graphite-Li cells with this new electrolyte provided evidence of cointercalation behavior and formation of t-GICs. They also demonstrated excellent cyclability at larger current densities. For example, the capacity retention reached ~92%, ~88%, and ~96% after 400 cycles. The average Coulombic efficiency (CE) – a measure of reversibility at the graphite anode – approached 100% after the first cycle. In contrast, DME pairing with LiFSI salt triggered rapid capacity fading.
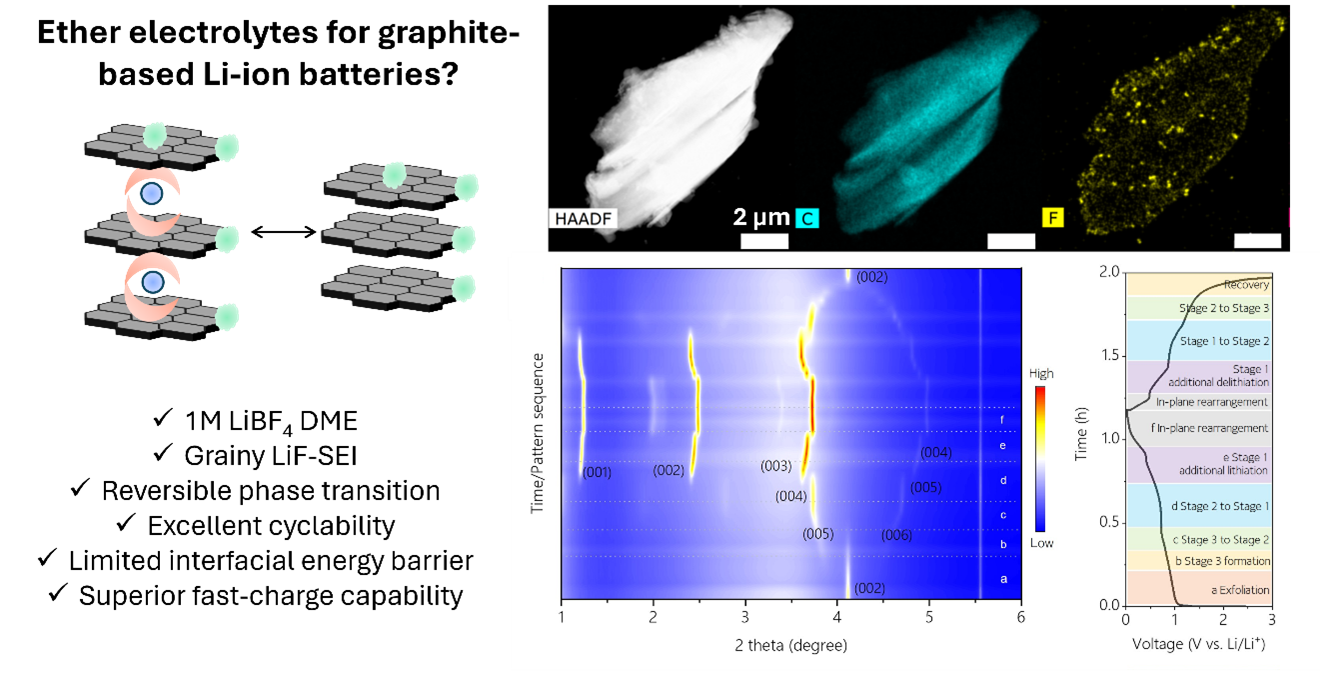
Structural studies including X-ray diffraction (XRD) and coherent X-ray multicrystal diffraction (CMCD) were performed at the APS beamlines. These advanced characterization methods demonstrated the reversible and monotonous phase transformation of cointercalated graphite. The researchers identified different phases of t-GICs with structures similar to Na-ion counterparts, which are known to be stable. All Li-ether could be extracted from the graphite during the delithiation process. Consequently, the graphite was not destroyed, but works reversibly.
Scanning-transmission electron microscopy and energy-dispersive X-ray spectroscopy revealed that the SEI on graphite in batteries using LiBF4 DME was made of LiF nanoparticles of sizes ranging from about 50 to 150 nm, mostly along the graphite’s edge plane. Rather than causing a runaway reduction reaction seen previously with ether-based solvents, reduction in of LiBF4 DME ceased after the first cycle, limiting degradation of the graphite anode. This self-terminating mechanism explains the high capacity retention and CE of the Li-graphite cells using the new electrolyte. In contrast, homogenous and dense SEI formed by LiFSI blocked the Li-ether cointercalation.
Together, the study authors say, these findings show that in contrast to conventional wisdom, Li-ether cointercalation can operate reversibly with the right choice of electrolyte, even at large current densities. They add that these findings set the stage for creating LIBs that can operate under extreme conditions using the cointercalation mechanism. They also lend some insight into the mechanism behind ether-based solvents in sodium and potassium batteries.
Enlightened by this study, a second study by the Virginia Tech researchers discovered a new cointercalation mechanism that enables even better cyclability, fast-charge ability, and low-temperature performance. They demonstrated a spontaneous reaction between binary-GICs and THF solvent concurrently during initial discharging. Operando X-ray and electrochemical analyses performed at the APS beamline 17-BM and 12-ID-D revealed that the graphite anode achieved unprecedented fast charging (1 min), low-temperature performance (-40 °C), and ultralong lifetimes exceeding 10,000 cycles. These two consecutive works clarify the confusion on ether electrolyte/graphite interface, resolve the stability issue of t-GICs, and demonstrate the feasibility of applying ether electrolytes for LIBs under extreme conditions. – Christy Brownlee
See: D. Xia1, H. Jeong2,3, D. Hou2,4, L. Tao1, T. Li2, K. Knight1, A. Hu1, E.P. Kamphaus2, D. Nordlund5, S. Sainio5, Y. Liu2, J.R. Morris1, W. Xu2, H. Huang1, L. Li2, H. Xiong1, L. Cheng2, F. Lin1, “Self-terminating, heterogenous, solid-electrolyte interphase enables reversible Li-ether cointercalation in graphite anodes,” PNAS 121 (5) e2313096121 (January 2024)
Author affiliations: 1Virginia Tech; 2Argonne National Laboratory; 3Gachon University; 4Boise State University; 5SLAC National Accelerator Laboratory.
The work was supported by Institute for Critical Technology and Applied Science at Virginia Tech and the Sun Grant program of the National Institute of Food and Agriculture, USDA, USA. The computational research was supported by the Joint Center for Energy Storage Research (JCESR), a U.S. Department of Energy (DOE), Energy Innovation Hub. We gratefully acknowledge use of the Bebop or Swing or Blues cluster in the Laboratory Computing Resource Center at Argonne National Laboratory. D.H. and H.X. acknowledge the support by the U.S. DOE, Office of Science, Office of Basic Energy Sciences program under Award Number DE-SC0019121. Work performed at the Center for Nanoscale Materials and Advanced Photon Source, both U.S. DOE Office of Science User Facilities, was supported by the U.S. DOE, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. Graphite was produced at the U.S. DOE CAMP (Cell Analysis, Modeling and Prototyping) Facility, Argonne National Laboratory, which is fully supported by the DOE Vehicle Technologies Program within the core funding of the Applied Battery Research for Transportation Program.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science
.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
