 Many disease-causing bacteria secrete toxins. Some of these toxins directly target human cells while others target the helpful symbiotic bacteria that protect the human body. The type VII secretion system is a process used by many of these bacteria to export toxins. One large family of toxins frequently exported through this export process has a distinguishing sequence motif comprised of the amino acids leucine and glycine (referred to as LXG).
Many disease-causing bacteria secrete toxins. Some of these toxins directly target human cells while others target the helpful symbiotic bacteria that protect the human body. The type VII secretion system is a process used by many of these bacteria to export toxins. One large family of toxins frequently exported through this export process has a distinguishing sequence motif comprised of the amino acids leucine and glycine (referred to as LXG).
Our current understanding of these toxic LXG proteins and the other proteins involved in this export process is incomplete. A recent study by scientists at McMaster University and the U.S. Department of Energy’s (DOE) Argonne National Laboratory determined the three-dimensional structure of an LXG toxin used by Streptococcus intermedius and ascertained which components of the toxin are necessary for export by the type VII secretion system, offering insights into combating the deleterious effects of thousands of similar toxins found in many disease-causing bacteria.
Streptococcus intermedius is a common, disease-causing bacteria with a thick layer of peptidoglycan reinforcing its outer membrane and protecting the bacterium. Bacteria with this fortified membrane are called Gram-positive. This reinforced outer membrane makes it difficult for bacteria to export toxins. To overcome this, Streptococcus intermedius uses a type VII secretion apparatus, anchored in the bacteria's membrane, which recognizes and exports the toxin molecules.
However, how the system recognizes toxins is not well understood. The exported LXG protein consists of two main parts: the LXG domain and the toxin domain. (A domain in a protein is a portion of the molecule which folds independently of the other portions and forms a stable globular unit.) The function of the toxin domain varies depending on the type of LXG protein but in all cases its purpose is to inhibit the growth of specific cells. By contrast, the function of the LXG domain has long remained unknown.
In this study, the team investigated an LXG protein called TelA to determine the function of the LXG and toxin domains. First, the team looked at the genes that encode for the TelA toxin. Based on previous studies of other LXG proteins, the team hypothesized that the genes for expressing the TelA toxin would be paired with two helper genes which encode for small proteins predicted to have a helical shape.
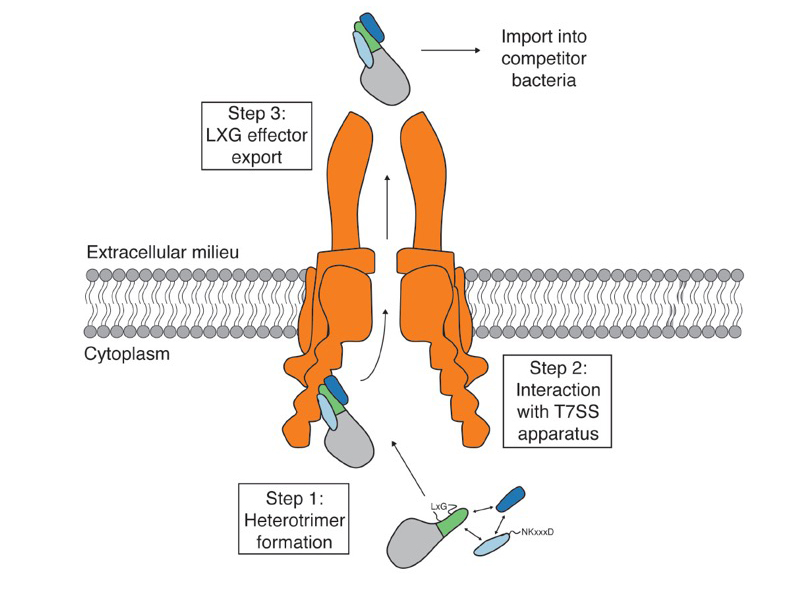
Researchers confirmed the presence of these helper genes adjacent to the TelA gene within the Streptococcus intermedius genome. They then expressed all three proteins and found that the two helper proteins were exported outside the bacteria along with the LXG toxin. The team also found that the TelA toxin was not exported if either of the two helper proteins were absent. Results showed that the TelA toxin and the two helper proteins form a three-part complex and that formation of this complex is required for toxin secretion. Figure 1 shows the model developed by the team to describe the export process for the TelA toxin.
To determine the structure for the protein complex---treating the TelA toxin and the two helper proteins as one unit---scientists performed X-ray crystallography using the Structural Biology Center beamline at 19-ID of the Advanced Photon Source, a DOE Office of Science user facility at Argonne, to produce three-dimensional images. The results showed that the LXG domain of the TelA toxin consists of two separate sub-domains shaped like lobes and that each of these lobes interacts with one of the two helper proteins.
The team next focused on the molecular details of the export process. They selectively removed portions from the protein complex to see which changes would result in failure to export the toxin. From their results, they concluded that the LXG domain is required for toxin domain export by the type VII secretion system. Additionally, the team concluded that certain amino acids within the LXG domain, including the leucine from which it derives its name, are key to the export process.
The team used the three-dimensional structure of the protein complex as an input for modeling software to characterize the structures of other full-length LXG proteins from other Gram-positive bacteria. Their results showed that other LXG proteins also have a three-part structure: two helper proteins in complex with the LXG domain. By incorporating results from research by others on type VII secretion systems, the team proposed that the structure of the all LXG toxins, when in complex with their helper proteins, is similar to a flagpole (LXG protein and the two helically-coiled proteins) with a waving flag (the toxin domain of the LXG protein).
By publishing the first three-dimensional structure of an LXG domain and demonstrating how said domain is required for toxin export by the type VII secretion systems of Gram-positive bacteria, this study opens opportunities for future research to interrupt these harmful processes and improve human health. – Mary Agner
____________________________________________________________________________________
See: T.A. Klein1, P.Y. Shah1, P. Gkragkopoulou1, D.W. Grebenc1, Y. Kim2, J.C. Whitney1, “Structure of a tripartite protein complex that targets toxins to the type VII secretion system,” PNAS 121 (3) e2312455121 (January 2024)
Author affiliations: 1McMaster University; 2Argonne National Laboratory.
We thank Tracy Palmer for sharing protocols and reagents, Giuseppe Melacini and Madoka Akimoto for access to and training on the SEC-MALS system, Dana Sowa for assistance with circular dichroism spectroscopy, and members of the Whitney Lab for helpful discussions. We sincerely thank the members of the Structural Biology Center (SBC) at Argonne National Laboratory for their help with data collection at the 19-ID beamline. The use of SBC beamlines at the Advanced Photon Source is supported by the U.S. Department of Energy (DOE) Office of Science and operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. T.A.K. and P.Y.S. are supported by Canada Graduate Scholarships from the Natural Sciences and Engineering Research Council of Canada. This work was supported by a project grant (PJT-173486) from the Canadian Institutes of Health Research. J.C.W. is the Canada Research Chair in Molecular Microbiology and holds an Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
