The past two decades have seen approximately 240 million reported cases of malaria annually, signaling a desperate need for an effective malaria vaccine.
Now, an international team has found that binding mode and cross-reactivity are as important to inhibitory function as antigen binding affinity. Using the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, to solve the structures of a whopping 22 crystals of 12 monoclonal antibodies (mAbs) bound to antigen peptides, they found that one of the four binding modes, combined with strong cross-reactivity, led to highly inhibitory antibody activity.
This work is part of the research team’s multiyear effort to develop a combination vaccine that targets vulnerabilities at different stages of the parasite’s life cycle. It is detailed in Cell Reports.
Most research to discover an effective antibody aims to uncover two characteristics: how well (and how quickly) an antibody binds with the antigen, and how effective the antibody is at inhibiting the parasite. In many cases, the antibodies that bind the best are also the most inhibitory. If the researchers are lucky, further study can reveal the biophysical mechanisms behind the antibody’s inhibitory effectiveness.
In this study, the researchers aimed to assess the efficacy of antibodies encoded by the IGHV3-33 gene. These antibodies are highly prevalent in the immune response against Pf circumsporozoite protein (PfCSP)—the most abundant surface protein on the deadliest malarial parasite, Plasmodium falciparum, and the antigen used here.
To begin, the team elicited monoclonal antibodies (mAbs) in mice whose own antibody genes had been replaced by a human gene repertoire. The mice were immunized with a long stretch of repeating amino acids from the PfCSP antigen’s junction and central domains. The researchers isolated the cells that produced the mAbs and sequenced the cells to understand the antibody response.
What they discovered surprised them. Rather than a strong correlation between binding affinity and inhibition, they found that between 12 antibodies that bound the PfCSP antigen equally well, some showed strong inhibition while others didn’t. That led the team to hypothesize that antibody efficacy was more complex than previously thought and that something other than binding affinity, such as structural characteristics, may be driving the principles of inhibition.
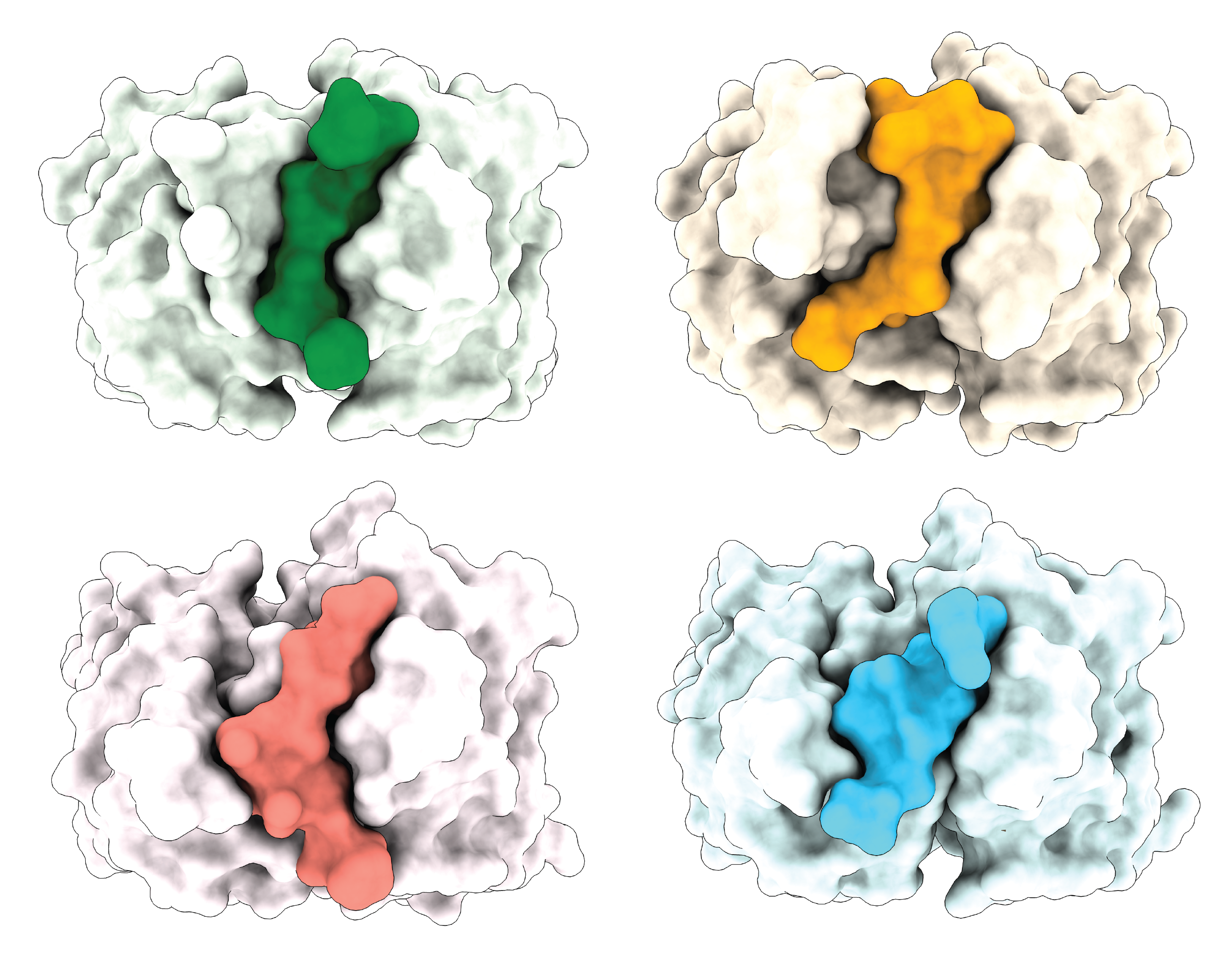
In pursuit of their goal, the team solved 22 crystal structures of 12 antibodies bound to PfCSP peptides, using beamlines 23-ID-B and 23-ID-D at the APS—a massive expenditure of resources and effort which, they hoped, would lead to meaningful conclusions beyond a one-antibody-one-antigen study.
The trove of data revealed that the antibodies bound to the antigen in four different binding modes, or conformations. The different binding modes altered the shape of the epitope and enabled the antibody to recognize many of the repeating amino acid motifs in the antigen’s junction and central domains. That means numerous antibodies can recognize and cluster around different motifs in a single antigen. In this unusual way, the antibodies carry high inhibition. The most effective antibodies typically assumed one particular conformation that conferred both strong cross-reactivity to the different repeat motifs and potent inhibitory efficacy.
The plethora of crystal structures revealed something else, too: a surprising diversity within this antibody family, giving it numerous different binding profiles. One antibody with a particularly unique binding mode that hadn’t been observed in any previous studies had high inhibition even though it had low cross-reactivity. This indicates that there is still more to learn about how different antibodies target the PfCSP antigen to inhibit the malaria parasite.
This international, multi-institutional, multi-disciplinary collaboration has been ongoing for many years, with one goal in mind: to eradicate malaria. The task is enormous: the Plasmodium falciparum has a very complex life cycle and expresses up to about 1200 proteins at any given life stage. A vaccine targeting only one protein addresses only one of the parasite’s life stages. If the immune system doesn't block that stage, the parasite can move forward.
The team is hoping to target the parasite in many different places. In addition to PfCSP, they’re looking at protein targets from the parasite’s other life stages as well. Additionally, now that this study has shown which characteristics make the most effective antibodies, the team can ponder how to present the antigen in a way that stimulates the immune system to make these high-efficacy antibodies. Their goal is simple but profound: to create a combination vaccine that can raise enough immune pressure to collapse the pathogen all the way from infection to malaria burden to transmission, moving toward complete elimination of malaria. – Judy Myers
____________________________________________________________________________________
See: E. Thai1,2,12,, R. Murugan3, S. Binter4,5, C. B. Aschner1, K. Prieto1, A. Kassardjian1,6, A. S. Obratsova3,7, R. W. Kang1,6, Y. Flores-Garcia8, S. Mathis-Torres8, K. Li9, G. Q. Horn9, R. H. C. Huntwork9, J. M. Bolscher10, M. H. C. de Bruijni10, R. Sauerwein10, S. M. Dennison9, G. D. Tomaras9, F. Zavala8, P. Kellam4,5,11, H. Wardemann3, J-P Julien1,2,6, “Molecular determinants of cross-reactivity and potency by VH3-33 antibodies against the Plasmodium falciparum circumsporozoite protein,” Cell Reports 42, 11 (November 2023)
Author affiliations: 1Hospital for Sick Children Research Institute; 2Department of Biochemistry, University of Toronto; 3Geman Cancer Research Center; 4Kymab Ltd./Sanofi; 5RQ Biotechnology Limited; 6Department of Immunology, University of Toronto; 7University of Heidelberg; 8Johns Hopkins Bloomberg School of Public Health; 9Duke University; 10TropIQ Health Science; 11Imperial College London.
F.Z.’s research is funded by the Bill and Melinda Gates Foundation (INV-001763). F.Z. and Y.F.-G. also thank the Bloomberg Philanthropies for continuous support and the insectary facilities of the Johns Hopkins Malaria Research Institute. We thank M. Abraha and M. Reichartz for SPR technical support and S. Mudrak and V. Bekker for program management support. We thank Nexelis for antibody serum measurements. HC-04, human hepatocytes, MRA-975 were obtained through BEI Resources, NIAID, NIH, contributed by Jetsumon Sattabongkot Prachumsri. This work was undertaken in part thanks to funding from the Bill and Melinda Gates Foundation (INV-008866 to J.-P.J. and H.W.; OPP1159947 to Š.B. and P.K.; and INV-008612 and INV-043419 to G.D.T.) and was supported by the CIFAR Azrieli Global Scholar program (to J.-P.J.), the Ontario Early Researcher Award program (to J.-P.J.), and the Canada Research Chair program (to J.-P.J.). E.T. was supported by a Vanier Canada Graduate Scholarship, C.B.A. by a Hospital for Sick Children Restracomp Postdoctoral Fellowship and a Banting Postdoctoral Fellowship, and A.K. by an Ontario Graduate Scholarship (OGS). We thank DKFZ/Heidelberg University/European Molecular Biology Laboratory Chemical biology core facility, in particular P. Sehr, for technical assistance and services. The BLI instrument was accessed at the Structural and Biophysical Core Facility, The Hospital for Sick Children, supported by the Canada Foundation for Innovation and Ontario Research Fund. X-ray diffraction experiments were in part performed using beamlines 23-ID-B and 23-ID-D at GM/CA@APS, which has been funded by the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006 and P30GM138396). This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract DE-AC02-06CH11357. The Eiger 16M detector at GM/CA-XSD was funded by NIH grant S10 OD012289. X-ray diffraction experiments were also performed using beamline AMX-17-ID-1 at the National Synchrotron Light Source II, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract DE-SC0012704. The Center for BioMolecular Structure (CBMS) is primarily supported by the National Institutes of Health, National Institute of General Medical Sciences (NIGMS) through a Center Core P30 grant (P30GM133893) and by the DOE Office of Biological and Environmental Research (KP1607011). X-ray diffraction experiments were also performed using beamline CMCF-ID at the Canadian Light Source, a national research facility of the University of Saskatchewan, which is supported by the Canada Foundation for Innovation (CFI), the Natural Sciences and Engineering Research Council (NSERC), the National Research Council (NRC), the Canadian Institutes of Health Research (CIHR), the Government of Saskatchewan, and the University of Saskatchewan.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
