Converting hydrocarbon gases into more valuable forms can help fight the buildup of atmospheric carbon that causes global warming, as well as providing a source of useful chemicals. Researchers have been searching for catalysts to make the process of reacting oxygen with such hydrocarbons more efficient. Now a group of scientists using the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, have demonstrated a catalyst that uses a ready source of oxygen and operates at near-ambient temperatures.
Many oxidation reactions rely on oxygen contained in some other molecule, such as hydrogen peroxide. But using dioxygen that’s readily available from the air would remove steps and simplify the process. Nature has already figured out how to do this, with enzymes containing, for example, non-heme iron–a form of iron that exists in plant tissues—that can activate dioxygen for hydrocarbon-oxygenation reactions. One such enzyme is taurine dioxygenase (TauD). TauD and its related dioxygenases are highly reactive because during the reaction they form a high-spin Fe(IV)=O species.
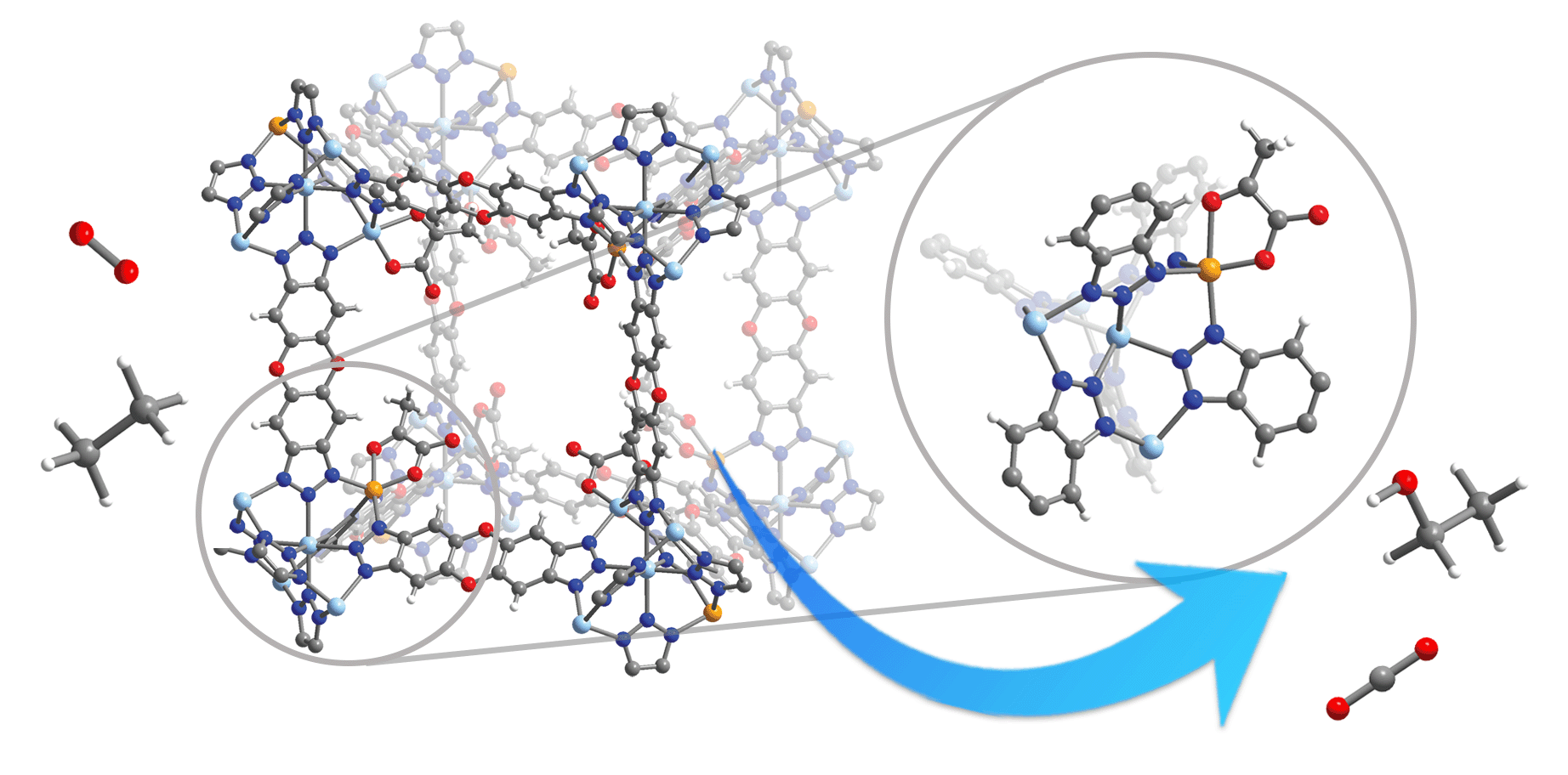
To mimic TauD, a team of researchers created a metal–organic framework (MOF), a porous structure made of metal nodes – in this case zinc and iron – and linked by organic molecules. Such MOFs can be easily tuned to specific needs by changing their chemical structure. The particular one created by the researchers was well suited to the catalytic oxygenation of hydrocarbons at roughly room temperature. Such low-temperature catalysis reduces the energy needed to run the reaction and makes the process more environmentally friendly than methods that require high temperatures.
The team tested their MOF by using it to turn ethane into ethanol through stoichiometric conversion and cyclohexane into cyclohexanol and cyclohexanone through catalytic oxygenation. The setup allowed the researchers to observe intermediates formed during the process, and they were able to spectroscopically characterize the Fe(IV)=O species.
To characterize their material, researchers performed powder X-ray diffraction at beamline 17-BM-B at the APS. That allowed them to take their starting material and treat it with oxygen at different temperatures, then check to see that the material remained crystalline at each stage. Such stability is important for a material being used as a catalyst in hydrocarbon oxygenation. They also performed nuclear resonance vibrational spectroscopy at beamline 3-ID-B at the APS. That allowed them to study the local structure of the Fe(IV)=O intermediate, and to confirm that it was that species.
Researchers also conducted studies at other facilities that helped round out the picture. Single-crystal X-ray diffraction analysis at the Advanced Light Source, a DOE Office of Science user facility at DOE’s Berkeley National Laboratory, let them examine the structure of the MOF. It showed that the structure was the same as it was in the powder diffraction work. Additionally, applied magnetic field Mössbauer spectroscopy at the Max Planck Institute for Chemical Energy Conversion in Mülheim and iron Kβ X-ray emission spectroscopy, performed at the BESSY-II light source at Helmholtz-Zentrum Berlin, helped confirm the high-spin state of the Fe(IV)=O intermediate. Diffuse reflectance infrared Fourier transform spectroscopy, performed on a custom-built setup in the researchers’ lab, provided additional information on the vibrational modes of the system.
Having demonstrated that their MOF works for hydrocarbon oxygenation, the researchers hope to extend its reactivity to other gases that may be more relevant for turning hydrocarbons into value-added alcohols, such as methanol. The work provides a foundation for developing new iron-containing MOFs to be used as catalysts that are similar to natural metalloenzymes in their reactivity.
Various state-of-the-art spectroscopic techniques, including the nuclear resonant vibrational spectroscopy (NRVS) at beamline 3-ID of the Advanced Photon Source, enabled the study of the nature of the material by isolating reactive sites and the reactive iron-oxo species. Such studies will benefit from the ongoing upgrade to the APS that will deliver even brighter X-ray beams in the near future.
– Neil Savage
____________________________________________________________________________________
See: K. Hou1,2, J. Börgel1,2, H. Z. H. Jiang1, D. SantaLucia3,4, H. Kwon1, H. Zhuang1, K. Chakarawet5, R. C. Rohde1, J. W. Taylor1, C. Dun2, M. V. Paley1,2, A. B. Turkiewicz1, J. G. Park1, H. Mao1, Z. Zhu1,2, E. E. Alp6, J. Zhao6, M. Y. Hu6, B. Lavina6,7, S. Peredkov3, X. Lv1, J. Oktawiec8, K. R. Meihaus1, D. A. Pantazis4, M. Vandone9, V. Colombo9,10, E. Bill3, J. J. Urban2, R. D. Britt5,1, F. Grandjean11, G. J. Long11, S. DeBeer3, F. Neese4, J. A. Reimer2,1, J. R. Long1,2, “Reactive high-spin iron(IV-oxo sites through dioxygen activation in a metal-organic framework,” Science 382 6670 547-553 (2023).
Author affiliations: 1University of California Berkeley; 2Lawrence Berkeley National Laboratory; 3Max Planck Institute for Chemical Energy Conversion; 4Max-Planck-Institut für Kohlenforschung; 5University of California Davis; 6Argonne National Laboratory; 7University of Chicago; 8Northwestern University; 9University of Milan; 10Consorzio Interuniversitario Nazionale per la Scienzia e Tecnologia dei Materiali (INSTM); 11University of Missouri.
This research was supported by the US Department of Energy Office of Basic Energy Sciences under award DE-SC0019992. Single-crystal X-ray diffraction data were collected at beamlines 11.3.1 and 12.2.1 of the Advanced Light Source at Lawrence Berkeley National Laboratory, a user facility supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences & Biosciences Division Heavy Element Chemistry Program under contract DE-AC02-05CH11231. Synchrotron powder X-ray diffraction data were collected on the 17-BM-B Beamline at the Advanced Photon Source, a US Department of Energy Office of Science User Facility operated by Argonne National Laboratory. NRVS data was collected at beamline 3-ID-B at the Advanced Photon Source. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences (contract DE-AC02-06CH11357). Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, US Department of Energy (contract DE-AC02-05CH11231). Instruments in the UC Berkeley College of Chemistry NMR facility are supported in part by NIH S10OD024998. EPR spectroscopic studies were funded by the National Institutes of Health (NIH 1R35GM126961 to R.D.B.). R.D.B. acknowledges support from the Miller Institute of Basic Research in Science, University of California, Berkeley. The computing cluster at the Molecular Graphics and Computation Facility at UC Berkeley is supported by NIH S10OD023532. The Fe Kβ XES measurements were carried out at the PINK beam line at the BESSY II electron storage ring operated by the Helmholtz-Zentrum Berlin für Materialien und Energie. D.J.S., S.P., E.B., D.A.P., S.D., and F.N. acknowledge the Max-Planck-Gesellschaft for funding. J.B. acknowledges the Deutsche Forschungsgemeinschaft for a Postdoctoral Research Fellowship. R.C.R. acknowledges the NASA Space Technology Graduate Research Opportunities fellowship. J.O. acknowledges the National Institute of General Medical Sciences for a Postdoctoral Research Fellowship under award F32GM143925. V.C. and M.V. acknowledge the Italian Ministry of University and Research for a Ph.D. fellowship awarded to M.V. and funding through PRIN2017 program (Project “Moscato” n° 2017KKP5ZR_004).
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
