Defects in the crystal structure of semiconductors are necessary for the operation of transistors, yet certain defect types can harm the electrical performance of these materials. The same is true of cathodes in batteries: Certain defects can make it easier for ions to move in and out of these materials and thus improve their performance, while others can block ion movement and degrade these materials, harming performance over time.
Complicating this issue is the transient nature of many defects in cathodes, causing them to arise only for a brief time during charging/discharging and then self-heal, which makes them difficult to detect. Thus, methods aimed at identifying defects in cathodes must be able to assess their crystal structures during active charging/discharging, and in three dimensions, which is pivotal for the layered nature of these materials.
One such technique, known as operando X-ray Bragg coherent diffraction imaging (BCDI), has demonstrated sufficient resolution to detect transient defects in lithium-ion batteries (LIBs). Although scientists have also identified transient defects in the cathodes of sodium ion batteries (SIBs) – a rapidly evolving, safe, sustainable, and inexpensive energy storage solution for large-scale applications – mechanisms behind their formation and dynamics were unclear.
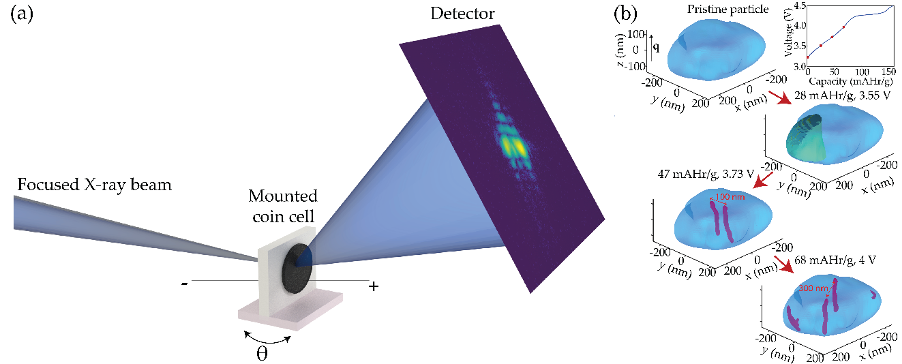
Using the 34-ID-C beamline at the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, researchers demonstrated the utility of using BCDI to track the evolution of defects in two different SIB cathode materials made of sodium, nickel, manganese, and oxygen in different proportions, providing different crystal structures. Their results showed a unique pattern of transient defects in these materials, results that could eventually help scientists engineer longer lasting and better performing SIB cathodes.
The researchers initially performed BCDI on working SIBs with one configuration of cathodes made of the above elements. Imaging showed that the nanoparticles that make up this material have a plate-like shape. During charging, the researchers observed the formation of screw dislocation loops, areas in which a plane of atoms shifts away from its normal location in the crystal structure, causing the surrounding layers to attach to each other improperly. These defects formed at antiphase domain boundaries, interfaces between relatively displaced crystal regions.
Additional results from BCDI showed that these defects arose in a heterogeneous fashion in this material. They also preferentially occurred perpendicular to the material’s layers. This contrasts with defects observed in other layered cathode materials such as those used in LIBs, which tend to occur between layers. The perpendicular orientation of transient defects in this material could potentially improve its performance by creating channels for ions to penetrate into the crystal structure.
To better understand sodium ion diffusion, the researchers uses their BCDI results to estimate the gradient of sodium ions that developed from the surface to the interior of the crystal structure during charging, showing an ionic gradient near the domain boundary and opening a way to study the kinetics of batteries during active use.
The researchers compared these findings to those derived from BCDI on batteries made with another cathode material, made from a different proportion of elements. This material also showed similar transient defect types. However, unlike the heterogeneous manner in which the first material’s defects arose, these tended to occur homogenously and were delayed until an abrupt shift in this material’s crystal structure.
Together, the researchers say, these findings offer new insights on how transient defects form and evolve in SIB cathode materials. Characteristics ranging from nanoparticle shape to material composition could be tailored to affect how transient defects arise and self-heal, optimizing the performance of SIBs. – Christy Brownlee
___________________________________________________________________________
See: O.Y. Gorobtsov1, H. Hirsh2, M. Zhang2, D. Sheyfer3, L.H.B. Nguyen2, S. D. Matson1, D. Weinstock1, R. Bouck1, Z. Wang1, W. Cha3, J. Maser3, R. Harder3, Y.S. Meng3,4, A. Singer1, “Operando interaction and transformation of metastable defects in layered oxides for Na-ion batteries,” Adv. En. Mat. 13 21 2203654 (2023)
Author affiliations: 1Cornell University; 2University of California San Diego; 3Argonne National Laboratory; 4University of Chicago.
The work at Cornell was supported by the National Science Foundation un- der Award Number (CAREER DMR 1944907). The work at UC San Diego was supported by the National Science Foundation (NSF) through the Partnerships for Innovation (PFI) grant IIP-2044465. The SEM analysis in this work was performed at the San Diego Nanotechnology Infrastructure (SDNI), a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation (grant ECCS1542148). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facil- ity, operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02- 06CH11357
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
