Supercrystals are crystalline assemblies of colloidal nanoparticles that generally form only when the strength of attractive interparticle interactions are relatively weak. This causes nanoparticles to reversibly bind and unbind during growth to rearrange into thermodynamically preferred lattice positions. These materials represent a unique class of crystalline structures because both the particle cores and their crystal symmetries can be tuned independently, which in turn controls their resulting electronic, magnetic and optical properties.
These properties, as well as their performance in applications such as catalysis and gas sorption, can be affected by imperfections in their crystal structure. Crystal defects can also provide clues into supercrystals’ growth mechanisms. One way to reliably form supercrystals is by using DNA as a bonding agent between nanoparticles, designing complementary regions of DNA that allow particles to assemble into lattices. Building stronger interactions into these complementary regions disrupts the nanoparticle rearrangement process, a scenario thought to introduce defects.
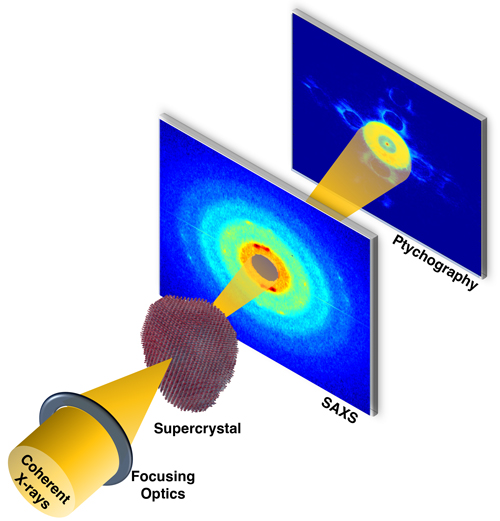
Although studies have suggested that such defects are prevalent in DNA-assembled supercrystals, direct observation has been challenging due to a lack of experimental techniques. For example, various electron microscopy approaches suffer from limited depth into the crystal interior, the presence of artifacts, or the destruction of the sample. Using the 12-ID-C beamline at the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science user facility at DOE’s Argonne National Laboratory, researchers from Northwestern University and Argonne used a new approach: a combination of X-ray ptychography and small-angle X-ray scattering (SAXS) to view defects in DNA-assembled supercrystals. Their findings revealed new insights into the growth mechanisms of these intriguing materials.
The team built supercrystals by functionalizing nanoparticles of varying sizes, ranging between 40 and 80 nanometers, with dense shells of DNA classified as either short (about 23.8 nanometers long) or long (about 34.5 nanometers long). Mixing a set of particles with complementary DNA caused these particles to aggregate. When the researchers heated these aggregates above their DNA melting temperatures and then cooled them to room temperature, they formed crystalline assemblies with sizes ranging from one micrometer to tens of microns. They then embedded these supercrystals in silica, allowing them to be visualized using X-ray ptychography and SAXS.
These techniques showed that all the samples formed body-centered particle arrangements. Samples embedded in silica exhibited compressed face-face distances, with the lattice shrinking more or less in proportion to the length of the DNA. However, the ultimate sizes of the supercrystals increased with the size of the particles used, with smaller particles forming smaller supercrystals and vice versa.
More subtly, the length of DNA also affected the quality of the resulting supercrystals. Scanning electron microscopy showed that supercrystals assembled with long DNA strands showed better-faceted exteriors compared to those made with short DNA relative to the same core size. These data were confirmed with ptychography, which not only showed better-faceted surfaces in the long DNA samples but also attachment of smaller supercrystals on mother supercrystal surfaces in the short DNA samples, often with different crystal orientations.
Ptychography also revealed substantial interior defects in almost all the samples, including edge dislocations and domain boundaries, with samples prepared with the short DNA strands having a higher density of defects. These types of defects suggest that these DNA-assembled supercrystals grew by aggregation of smaller nanocrystals, followed by rearrangement of nanoparticles. The higher prevalence of defects in supercrystals with shorter DNA linkers appeared to be due to a higher potential for attraction, the study authors suggest, which inhibited bound particles from unbinding and rebinding into ideal positions in the crystal lattice.
This proposed mechanism was reinforced by ptycho-tomograms of supercrystals with the smallest cores. In large area views, these samples showed many small crystals connected in random directions like a necklace, with the size of each single domain smaller than those with larger cores.
Together, the researchers suggest, these findings indicate that DNA strands grafted onto nanoparticles prompt the growth of large supercrystals through the random attachment of smaller supercrystals formed through nucleation and growth, which often attach without matching lattice orientations. This discovery was made possible through this combination visualization technique. – Christy Brownlee
_____________________________________________________________________________
See: H. A. Calcaterra1, C. Y. Zheng1, S. Seifert1, Y. Yao2, Y. Jiang2, C. A. Mirkin1, J. Deng2, B. Lee2, “Hints of growth mechanism left in supercrystals,” ACS Nano 17, 16, 15999-16007 (2023)
Author affiliations: 1Northwestern University; 2Argonne National Laboratory
This material is based upon work supported as part of the Center for Bio-Inspired Energy Science, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award DE-SC0000989 (for nanoparticle synthesis) and the Air Force Office of Scientific Research award FA955022-1-0300 (for nanoparticle assembly and characterization). C. Zheng acknowledges support by the U.S. Department of Energy (DOE), Office of Science, Office of Workforce Development for Teachers and Scientists, Office of Science Graduate Student Research (SCGSR) program. The SCGSR program is administered by the Oak Ridge Institute for Science and Education (ORISE) for the DOE. ORISE is managed by ORAU under contract number DE-SC0014664. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of DOE, ORAU, or ORISE. H. Calcaterra acknowledges support by the National Science Foundation Graduate Research Fellowship Program grant (DGE-1842165). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility and is based on work supported by Laboratory Directed Research and Development (LDRD) funding from Argonne National Laboratory, provided by the Director, Office of Science, of the U.S. DOE under Contract No. DE-AC02-06CH11357. This work made use of the EPIC facility of Northwestern University’s NUANCE Center, which receives support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-1542205); the MRSEC program (NSFDMR-1121262) at the Materials Research Center; the International Institute for Nanotechnology (IIN) and the State of Illinois, through the IIN. B. Lee acknowledges support by the National Science Foundation under Grant No. NSF PHY-1748958. B. Lee expresses gratitude to Alex Travesset and other participants of the nanoassembly program at the Kavli Institute for Theoretical Physics (KITP) for their stimulating discussions.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
