Popular culture represents the human heart as sleekly curved and full of glitter at Valentine's Day, but anatomically the heart consists of muscle that contracts to provide blood to the body. And, like other muscles, anatomical hearts can develop conditions that impair their ability to contract. A more complete understanding of what occurs physically and biochemically within heart muscle would allow medicines to focus on specific muscle components or interactions to improve the heart's ability to contract.
Recent research by a team of investigators from the Illinois Institute of Technology and the University of Washington presents a more detailed description of the positional changes of the myosin proteins within the heart as they prepare for contraction, and demonstrates how the myosin's behavior directly affects the amount of force created during muscle contraction, revealing new focus points for medicines.
Muscles consist of long fibers in repeating sections. Each section of heart muscle is built from parallel strands of myosin and actin proteins. Myosin is a motor protein that converts the chemical energy stored in adenosine triphosphate, or ATP, to mechanical energy for muscle contraction. The thick strand of a myosin protein is called its tail, which connects to the protein's neck and two heads. When a muscle is relaxed, the myosin and actin proteins don't bind with each other and the myosin heads rest on the tail. During muscle contraction---a process stimulated by the presence of calcium ions---the heads of the myosin proteins bind to the actin proteins and tug on them, shortening the muscle fiber. Scientists hypothesize that the number of myosin heads which pull on the actin proteins to produce force during contraction can be modulated by the positional changes of the myosin heads in their resting state.
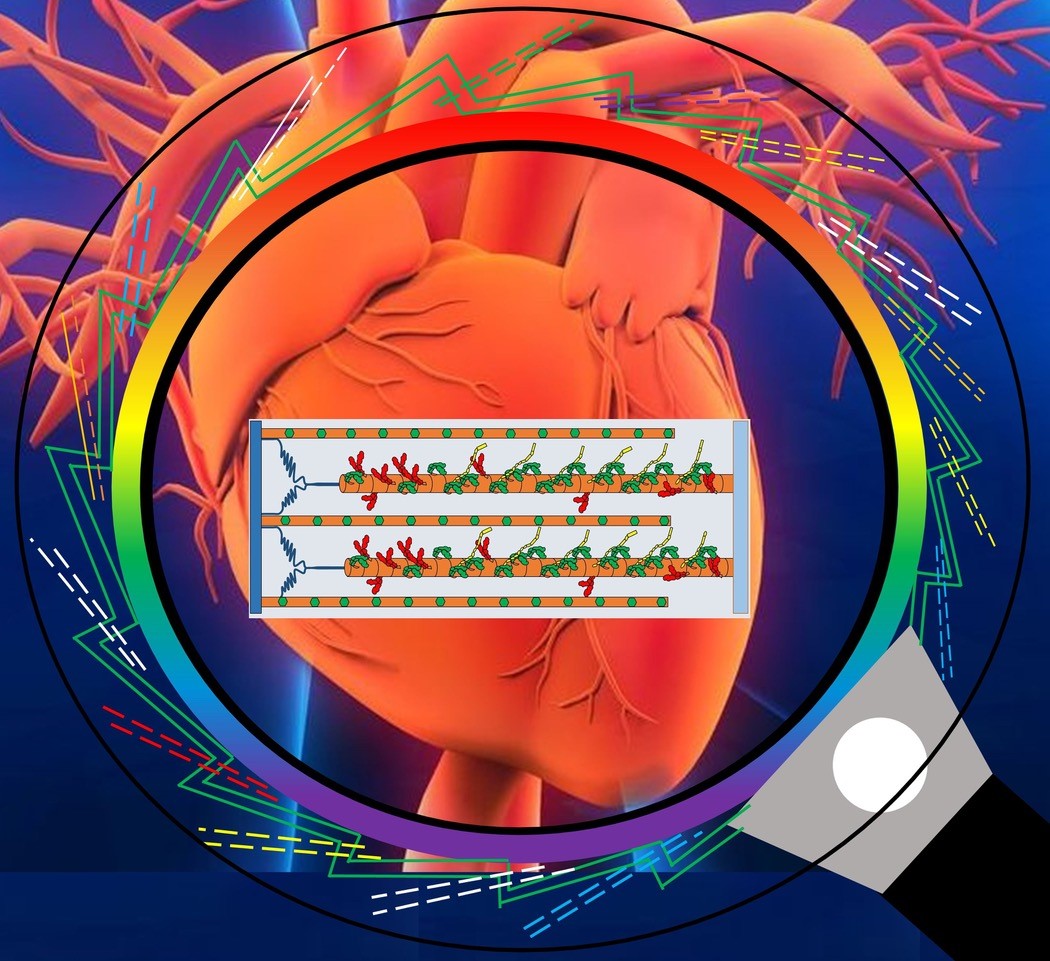
To test this hypothesis, the team used small-angle X-ray diffraction at the BioCAT beamline (18-ID) to image relaxed heart muscle from pigs in solution. Figure 1 depicts how X-ray diffraction acts as a super magnifying glass to show molecular motion within the heart. Each solution had a different concentration of 2-deoxy-adenosine triphosphate, or dATP, which provided an energy source for the myosin heads to move, allowing the team to image any changes in the myosin heads' positions.
To understand how the pre-contraction positional change of the myosin heads affects the amount of force produced during contraction, the team measured tension and relative stiffness of thin strips of a pig's left ventricle. The team also performed molecular dynamics simulations to examine the electrostatic interactions between chemical bonds at key sites on the myosin protein.
The team's mechanical measurements showed that the structural changes induced by changing the concentration of dATP under relaxing conditions correlated with greater force during contraction. Combining these results with molecular dynamics simulations allowed the team to understand how the electrically charged myosin and actin proteins interacted during the positional change of the myosin heads.
The myosin binds with actin for contraction but also creates energy within the fiber by contributing to the breakdown of ATP in the resting state. The team compared their results with those reported in previously-published journal articles. They found that the presence of dATP affects the rate at which myosin contributes to ATP breakdown differently than its presence affects the movement rate of myosin heads toward actin. The team also concluded that the amount of force produced when the myosin head tugs on the actin protein is informed by the head's positional changes rather than by its interactions helping to break down ATP under resting conditions.
Because the pre-contraction positional changes and the electrostatic interactions between myosin and actin are not currently utilized by available medicines, the team suggests new medicines that incorporate this information have potential for improving heart muscle contraction, a fairly sparkly possibility even outside of Valentine's Day. — Mary Agner
See: Weikang Ma1*, Timothy S. McMillen2, Matthew Carter Childers2, Henry Gong1, Michael Regnier2, Thomas Irving1, “Structural OFF/ON Transitions of Myosin in Relaxed Porcine Myocardium Predict Calcium-Activated Force,” PNAS 120 (5) e2207615120 (2023). DOI: https://doi.org/10.1073/pnas.2207615120.
Author affiliations: 1Illinois Institute of Technology, 2University of Washington
Correspondence: * wma6@iit.edu.
This research was supported by NIH grant R01 HL128368 (M.R.). This research used resources of the University of Washington Center for Translational Muscle Research, supported by the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases under Award Number P30AR074990 (M.R.) This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Use of the BioCAT beamline 18ID is supported by grant P30 GM138395 (T.I.) from the National Institute of General Medical Sciences of the NIH. The content is solely the authors’ responsibility and does not necessarily reflect the official views of the National Institute of General Medical Sciences or the NIH.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
