Uranium-niobium (U-Nb) alloys, used in a variety of energy and defense applications that require high density, ductility, and good corrosion resistance, comprise a highly complex, multiphasic system with a phase diagram well-established through decades of extensive experimentation. Such studies have closely studied the alloy's temperature-dependent phase transformation, growth kinetics, and microstructural evolution, but most of the work has focused on conditions below the monotectoid temperature (the isothermal reversible change of a solid phase to form two different solid phases during cooling) of 647° C. Experimenters used in situ and real-time synchrotron x-ray studies at the U.S. Department of Energy’s Advanced Photon Source (APS) to investigate the intriguing realm above this temperature, where phase and compositional homogenization occurs. Their work appeared in the Journal of Nuclear Materials.
Although the U-Nb system has been the subject of intensive study for years, relatively little attention has been given to the homogenization process above the monotectoid temperature, where a-U and g1-2 microstructures become a single-phase solid solution, gs, before melting. However, a better understanding of the dynamics and evolution of the homogenization phenomenon may have important implications for the manufacturing processes of U-Nb alloys.
In this work, the investigators studied a U-6Nb alloy because of its particular affinity for a wide range of applications. Time-resolved concurrent wide-angle (WAXS) and small-angle x-ray scattering (SAXS) studies were performed at the APS X-ray Science Division Materials Physics & Engineering Group’s 1-ID beamline at the APS. The studies centered on U-6Nb samples during four different heating and hold cycles for up to 50 minutes above the monotectoid temperature of 647° C, up to 750° C.
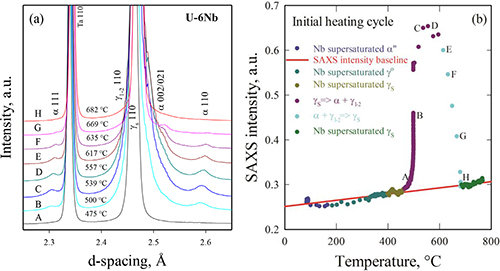
Distinct microstructures were evident in each of the cycles below the monotectoid temperature, but these began to homogenize during heating above that point. Time-resolved in situ WAXS data revealed the homogenization process. At first, a"® g0 ® gs reverse martensitic transformations were observed. This was followed by decomposition into a two-phase mixture of a and g1-2 with the growth of a-U made evident by the increase in intensity of a-U diffraction peaks while the g1-2 diffraction peaks remained weak near the gs shoulder. As the temperature of the mixture was further increased, a+g1-2 ® gs homogenization started to occur, approximately between 617° C and 635° C. With the disappearance of a-U at 682° C, the homogenization process was complete.
It was also revealed through the peak width of the gs phase that the phase and compositional homogenization process within the volume illuminated by x-rays (0.01 mm3) was completed within 60 seconds, consistent with interdiffusion homogenization of fine lamellae structures.
The researchers also found that the comparison of integrated SAXS intensities with WAXS data yielded complementary information on the chemical segregation and phase transformation process. For instance, the SAXS intensities revealed that a"® g0 ® gs is an athermal process under high heating rate. The SAXS intensities also showed that the initiation temperature for the a+g1-2 ® gs homogenization process is between 617° C and 635° C.
Further investigation of the homogenization process in the alloy samples aged at different times and temperatures showed two stages with different mechanisms. The first stage was marked with apparent thermal expansion as the lattice parameters increased with the g1-2 temperature. This changed in the second stage with reduction of Nb content in g1-2 and SAXS intensities. These stages appear to correspond with the a"+ g1-2 ® gs homogenization reaction. With continued heating above 682° C, the gs phase continued to replace a-U and g1-2 until complete homogenization was achieved. However, these pre-aged alloys showed some slight differences in the time before complete phase and compositional homogenization. The researchers attribute these differences to several effects arising from the initial alloy microstructures, including a-U content, the bcc phase of Nb, and lamellar structures.
These experiments provide an excellent demonstration of the great effectiveness of time-resolved in situ synchrotron SAXS and diffraction studies for probing the inner workings of the important U-Nb system. A more detailed understanding of the microstructural behavior of these alloys under various temperature and time regimes that such tools provide will allow them to be better tailored for their unique applications. ― Mark Wolverton
See: Jianzhong Zhang1, Erik B. Watkins1*, Donald W. Brown1, Bjorn Clausen1, Peter Kenesei2, and Jun-Sang Park2, “Time-resolved phase and compositional homogenization of segregated uranium-niobium alloys above the monotectoid temperature,” J. Nucl. Mat. 564, 153673 (2022). DOI: 10.1016/j.jnucmat.2022.153673
Author affiliations: 1Los Alamos National Laboratory, 2Argonne National Laboratory
Correspondence: * ebw@lanl.gov,
This work was supported by the U.S. Department of Energy (DOE) through the Los Alamos National Laboratory. Los Alamos National Laboratory is operated by Triad National Security, LLC, for the National Nuclear Security Administration of the U.S. DOE (Contract No. 89233218CNA0 0 0 0 01). The research presented in this article was supported by the Science Campaign Programs. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility at Argonne National Laboratory and is based on research supported by the U.S. DOE Office of Science-Basic Energy Sciences , under Contract No. DE-AC02- 06CH11357 .
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
