Never cheap, lithium (Li) is becoming increasingly more expensive as global supplies dwindle, boosting the cost of the rechargeable lithium-ion (Li-ion) batteries that power today’s electric vehicles, smartphones, and power tools. Li-ion batteries work by shuttling Li+1 ions between two electrodes (cathode and anode), with the positive charges of the ions contributing to the electrical energy the batteries store and discharge. Researchers are seeking to replace the Li ions in the batteries with cheaper, more abundant metallic ions that can also store more energy per ion owing to their two positive charges instead of lithium’s one, with magnesium ions (Mg+2) being a prominent candidate. Building on previous research, scientists tailored the promising solid solution spinel oxide MgCrMnO4 so that it would be free of defects, particularly Mg/Mn antisite inversion defects. Using the U.S. Department of Energy’s Advanced Photon Source (APS), they then characterized the structural, compositional, and electron-transfer (redox) properties of the optimized spinel oxide before and after cycling the material as an electrode in a Mg-ion battery. Their results are published in the journal Chemistry of Materials.
Successful development of Mg-ion batteries strongly depends on finding cathode materials possessing inner migration pathways (interstitial spaces) that (1) permit Mg ions to easily enter, occupy, and exit (intercalate and deintercalate) the materials at high voltage and all states of charge and (2) do not significantly damage the host materials in the process.
The researchers synthesized a series of solid-solution nanocrystalline MgCr2−xMnxO4 spinels containing varying proportions of chromium (Cr) and manganese (Mn) atoms. High-resolution synchrotron x-ray diffraction data collected at the X-ray Science Division (XSD) Structural Science Group’s 11-BM beamline at the APS, an Office of Science user faciltiy at Argonne National Laboratory. These data showed that an optimized version of MgCr1.5Mn0.5O4 was a near-ideal spinel configuration having only about 1% inversion defects without any segregated phases. In a 1% Mg/Mn antisite inversion, only 1% of the Mg lattice sites are occupied by Mn ions and vice versa. These misplacements at higher levels would create barriers to Mg2+ migrations and decrease the energy capacity of a Mg-ion battery made with the cathode material.
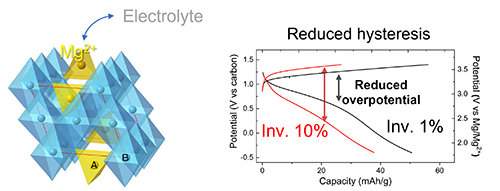
The electrochemical properties of the optimized MgCr1.5Mn0.5O4 spinel (∼1% inversion) were measured at 95° C after the spinel was inserted in a Mg half-cell battery as a working electrode. A charging capacity of ∼56 mA h/g was achieved at 1.4 V, corresponding to approximately 0.2 mol of Mg2+ equivalents reacting per mole of MgCr1.5Mn0.5O4, a proportion that was consistent with x-ray diffraction data. Subsequently, the cell showed a discharge capacity of ∼50 mA h/g. This was in contrast to a Mg half-cell battery made with a nonoptimized MgCr1.5Mn0.5O4 spinel oxide (∼10% inversion defects), which had a lower charge capacity (∼29 mA h/g) upon charging to 1.4 V. Upon subsequent discharge to −0.4 V, the half-cell achieved a capacity of ∼38 mA h/g. Beside the decrease in capacity of ∼14 mA h/g, a larger overpotential for re-intercalation was observed for the half-cell made from the nonoptimized spinel oxide (Fig. 1), that is, greater electrochemical hysteresis was seen, demonstrating the importance of minimizing the number of defects in the spinel’s structural configuration. While the reported capacities were low due to the small concentration of redox active Mn in the material, the results showed Li-ion cathode-like structure activity for the first time for a Mg-ion oxide cathode (Fig. 1).
The amount of Mg2+ that was deintercalated solely from the lattice upon charging was quantified by solid-state nuclear magnetic resonance (NMR) spin counting experiments using a spinel oxide made from the 25Mg isotope to gain additional signal and resolution. The 25Mg-enriched spinel, MgCr1.5Mn0.5O4, containing ∼10% of inversion defects, was operated as a battery electrode, revealing that approximately 0.25 mol of Mg2+ was removed upon charging.
Valence state changes at the Mn and Cr ions were investigated to understand the evolution of the electronic environment in the optimized MgCr1.5Mn0.5O4 spinel in response to Mg2+ intercalation and deintercalation. An Mn K-edge x-ray absorption near-edge structure experiment was performed at the Materials Research Collaborative Access Team (MR-CAT) insertion device beamline 10-ID at the APS. Mn LII,III-edge x-ray absorption spectroscopy (XAS) measurements were carried out at the XSD Magnetic Materials Group’s beamline 29-ID also at the APS. Cr LII,III edge XAS measurements took place at beamline 7-ID-1 of the U.S. DOE’s National Synchrotron Light Source II (NSLS-II) at Brookhaven National Laboratory. Bulk redox changes at Mn were detected upon intercalation and deintercalation of Mg2+, whereas the valence state of Cr remained unchanged.
The findings of the study enhance the understanding of Mg2+ transport within spinel oxide frameworks and provide conclusive evidence for bulk Mg migration in oxide lattices at high redox potentials with minimized electrochemical hysteresis. The experimental evidence emphasized the influence of structural defects, in this case inversion, on electrochemical Mg2+ activity and provided a design rule toward building a functional Mg cathode for a high-energy Mg-ion battery. ― Vic Comello
See: Bob Jin Kwon1*, Liang Yin1, Indrani Roy2, Noel J. Leon1, Khagesh Kumar2, Jae Jin Kim1, Jinhyup Han1, Jihyeon Gim1, Chen Liao1, Saul H. Lapidus1, Jordi Cabana1, and Baris Key1**, “Facile Electrochemical Mg-Ion Transport in a Defect-Free Spinel Oxide,” Chem. Mater. 34, 3789 (2022). DOI: 10.1021/acs.chemmater.2c00237
Author affiliations: 1Argonne National Laboratory, 2University of Illinois at Chicago
Correspondence: * bkwon@anl.gov; ** bkey@anl.gov
This work was intellectually led and primarily supported by the Joint Center for Energy Storage Research (JCESR) and Energy Innovation Hub funded by the U.S. Department of Energy (DOE) Office of Science-Basic Energy Sciences. I.R. and K.K. acknowledge support by the National Science Foundation under grants DMR-1809372 and CBET-18000357, respectively. This research used resources of the National Synchrotron Light Source II at 7-ID-1, a U.S. DOE Office of Science User Facility operated for the U.S. DOE Office of Science by Brookhaven National Laboratory under contract no. DESC0012704. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. DOE’s APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation’s first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state, and municipal agencies to help them solve their specific problems, advance America’s scientific leadership, and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
