An appealing variation of lithium-ion (Li-ion) batteries is the lithium-sulfur (Li-S) design. While Li-ion batteries often utilize cobalt or other expensive and potentially toxic metals as cathode, the sulfur used in Li-S batteries is abundant, cheap, and environmentally friendly. Furthermore, Li-S batteries potentially have two-to-three times higher energy densities than the other state-of-the-art Li-ion batteries. Unfortunately, the widespread adoption of Li-S battery technology has been hampered by several inherent problems. In particular, excessive migration of long-chain sulfur molecules, called polysulfides, leads to significant degradation in battery performance. Now for the first time, researchers have incorporated a porous silica material within experimental lithium-sulfur battery cells that greatly reduces polysulfide migration while increasing electrical output. Laboratory tests demonstrated that the new design substantially increased battery energy density and lifespan under realistic charge/discharge cycling. The experimental Li-S cells were further characterized using a number of techniques, including x-ray diffraction carried out at the U.S. Department of Energy’s Advanced Photon Source (APS). The scientists carrying out this research are confident their findings, which were published in the journal Nature Communications, will hasten the adoption of Li-S battery technology to meet the demands of a sustainable energy future.
Typically, each cell in a Li-ion battery has a graphite or similar carbon-based anode (the negative electrode), as well as a positive cathode incorporating cobalt or other metallic elements. Sandwiched between the anode and cathode is an electrolyte, usually consisting of a liquid or gel. During battery discharge, lithium ions travel from the anode through the electrolyte and into the cathode. As the battery is charged the lithium ions migrate back from the cathode to the anode.
A Li-S battery is also powered by the movement of lithium ions. The lithium ions initially reside in a lithium metal anode, and then migrate during discharge toward a sulfur cathode (the sulfur is mixed with other compounds that improve strength and conductivity). Although lithium-sulfur batteries have been around since the 1960s, the design has found only limited use due mostly to a shortened lifespan.
The limited Li-S lifespan can be traced to the migration of polysulfides from the sulfur-rich cathode to the lithium metal anode. This migration occurs because polysulfides dissolve into the electrolyte, leading to a concentration gradient between cathode and anode. The movement of these sulfur chains is known as polysulfide shuttling, and it can chemically corrode the lithium metal anode and degrade battery performance.
Polysulfide shuffling has been investigated for decades. One attempted solution has been the placement of a barrier, or interlayer, between the cathode and anode that blocks the polysulfide chains while still permitting the movement of lithium ions. Although interlayers can restrict polysulfide shuttling, they often tend to reduce the free flow of lithium ions during battery operation, which limits performance. Interlayers can also add significant weight to the battery.
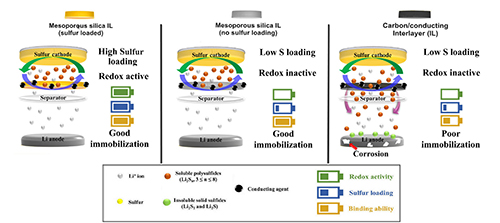
Interlayers that are chemically inert and designed only to block polysulfide shuttling are called inactive. By contrast, active interlayers exhibit electrochemical activity within the battery. For this study, researchers constructed an active interlayer designed to prevent polysulfide shuttling while enhancing battery performance without adding significant weight. They chose a mesoporous silica material with microscopic pockets measuring around 7 nanometers across. This mesoporous silica is very lightweight, and its pores provide innumerable spaces for loading sulfur. The resulting sulfur-impregnated interlayer not only blocks polysulfide migration, it also boosts the redox (reduction/oxidation) electrochemistry of the battery.
Three distinct silica mesoporous interlayers were created, loaded with either 30%, 50%, or 70% sulfur by weight. Additionally, the researchers produced silica mesoporous interlayers with no sulfur, as well as a carbon-based mesoporous interlayer. All these interlayers were then incorporated into experimental battery cells.
Testing revealed that only the sulfur-loaded mesoporous interlayers effectively blocked polysulfide shuttling while simultaneously boosting the cells' electrochemical activity (Fig. 1). The research team also characterized the structural and elemental changes to the experimental interlayers using various analytical methods, including x-ray diffraction studies performed at the APS X-ray Science Division Structural Science Group’s beamline 17-BM of the APS, an Office of Science user facility at Argonne National Laboratory. Overall, the mesoporous interlayer with 50% sulfur loading exhibited the best performance, retaining cell stability after 700 charge/discharge cycles under high electrical current loading.
These findings demonstrate a lightweight interlayer architecture that effectively blocks polysulfide shuttling while increasing battery energy density and lifespan. Coupled with other advances in, for example, the durability of the sulfur-rich cathode, it is anticipated that these new results will dramatically enhance the commercial viability of Li-S batteries. ― Philip Koth
See: Byong-June Lee1, Chen Zhao2, Jeong-Hoon Yu1, Tong-Hyun Kang1, Hyean-Yeol Park1, Joonhee Kang3, Yongju Jung4, Xiang Liu2, Tianyi Li2, Wenqian Xu2, Xiao-Bing Zuo2, Gui-Liang Xu2*, Khalil Amine2,5,6**, and Jong-Sung Yu1***, “Development of high-energy non-aqueous lithiumsulfur batteries via redox-active interlayer strategy,” Nat. Commun. 13, 4629 (2022). DOI: 10.1038/s41467-022-31943-8
Author affiliations: 1Daegu Gyeongbuk Institute of Science & Technology (DGIST), 2Argonne National Laboratory, 3Pusan National University, 4Korea University of Technology and Education (KOREATECH), 5Stanford University, 6Mohammed VI Polytechnic University
Correspondence: * xug@anl.gov, ** amine@anl.gov, *** jsyu@dgist.ac.kr
B.-J.L., T.-H. K., H.-Y.P., J.K., Y.J., and J.-S.Y. gratefully acknowledge financial support by the National Research Foundation (2019R1A2C2086770 and 2016M1A2A2937137) funded by the Ministry of Science, ICT & Future Planning. B.-J.L., T.-H. K., H.-Y.P., J.K., Y.J., and J.-S.Y. also thank the CCRF at DGIST and the KBSI at Daegu and Pusan for SEM, TEM, and XPS measurements. Research at the Argonne National Laboratory was funded by the U.S. Department of Energy (DOE), Vehicle Technologies Office. Support from Tien Duong of the U.S. DOE’s Office of Vehicle Technologies Program is gratefully acknowledged. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
