Scientists have shown how oxygen ions interact with a complex oxide, strontium cobalt oxide, causing it to switch from a metal to an insulator and back again. The researchers say that the techniques they used could be utilized for novel studies of other complex oxides, which have applications in many current and future technologies. The results, based on research in the Materials Science Division and Advanced Photon Source (APS), both at Argonne National Laboratory, and the Materials Science and Technology Division and Center for Nanophase Materials Sciences, both at Oak Ridge National Laboratory (ORNL), are published in Physical Review Letters.
Materials in which one can manipulate the proportion of oxide ions are considered good candidates for the development of devices that employ ionotronics (powerful tools and methods for narrowing the gap between conventional electronics and biological systems). Such ionotronic devices, which use ion migration rather than electron transfer to transport charge, could exhibit reversible metal-to-insulator transitions. This is a switch from a metal state with good electrical conductivity to an insulator, a material that supresses electrical conductivity. Materials that can transition between these two states are considered useful for a range of new electronic devices. In suitable ionotronic devices, the reversible metal-to-insulator transition could be triggered by the uptake or loss of oxygen.
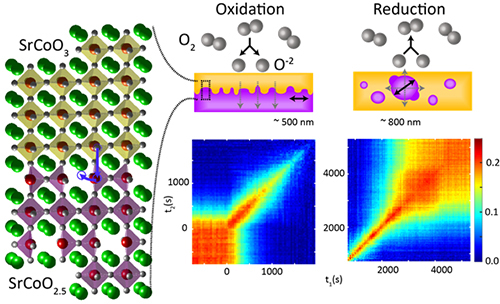
One material of interest is strontium cobalt oxide. This complex oxide can transition between two phases: the strontium cobalt oxide 2.5 phase (with the chemical formula SrCoO2.5), known as the brownmillerite phase, as it has the same crystal structure as the mineral brownmillerite, and the strontium cobalt oxide 3 (SrCoO3) or perovskite phase, which has the same structure as calcium titanium oxide, a mineral also known as perovskite.
In the brownmillerite phase, strontium cobalt oxide is insulating and antiferromagnetic, while the perovskite phase is metallic and ferromagnetic. When switching between these states, the oxide reorders itself and changes its crystal structure as it takes up or loses oxygen.
One use for this switch could be to write memories, with the conducting state as a 1 and the insulating state as a 0, for instance, but now with a continuum of memory-states in between determined by the amount of oxygen content in the material. As well as the metal-insulator transition, there could also be interest in the ferromagnetic-to-antiferromagnetic switch. While origin of this transition was recently explained by theorists at ORNL [1], to take advantage of the transition, it is helpful to understand its dynamics; that is, how quickly oxygen moves in and out of the different phases.
Materials scientists used two beamlines at the APS, an Office of Science user facility at Argonne National Laboratory, to probe the phase transition dynamics of strontium cobalt oxide using several different x-ray techniques. The researchers used X-ray Science Division (XSD) Chemical & Materials Science Group’s x-ray beamline 12-BM-B to perform x-ray near-edge absorption spectroscopy, and the XSD Dynamics & Structure Group’s beamline 8-ID-E to take synchrotron x-ray microdiffraction measurements and undertake x-ray photon correlation spectroscopy (XPCS). Theoretical work led by ORNL employed electronic structure methods, including both many-body quantum Monte Carlo (QMC) and density functional theory, to perform predictive atomistic simulations, which provided the mechanistic understanding of the kinetics of the phase-transition and was validated by these experimental measurements. Understanding the kinetics, helped inform the dynamics of the transition. The QMC effort at ORNL used QMCPACK and was supported by the U.S. DOE Center for Predictive Simulation of Functional Materials.
A 30-nanometer-thick layer of strontium cobalt oxide was grown on a substrate of strontium titanate using pulsed-laser deposition. After synthesis, the material was in the brownmillerite phase. The team introduced oxygen or nitrogen to oxidize or reduce the material, respectively, inducing phase changes from brownmillerite to perovskite and perovskite to brownmillerite.
Previous research has shown that the crystal lattices of the two phases are similar. The overall crystal shape is maintained during transition, with changes in the relative amounts of brownmillerite and perovskite states as the transition progresses through the material.
The team found that during oxidation―the switch from brownmillerite to perovskite―oxygen molecules broke down into ions and filled vacancies at the top of the brownmillerite structure. They then diffused into the film and the perovskite phase spread down through the material. But due to the nature of the oxygen dynamics, there were significant fluctuations at the boundary between the two phases―the change in phase did not spread evenly as it moved down through the material.
When nitrogen was introduced, oxygen vacancies formed in the perovskite phase, creating nuclei of brownmillerite. These then expanded in a three-dimensional manner driving excess oxygen out of the material. This expansion was much smoother, with little fluctuation at the phase boundaries, and over time the nuclei joined up.
The researchers also took advantage of coherence in the x-rays―properties that will improve greatly with the impending upgrade at the APS. New techniques exploiting coherence, such as XPCS, enabled the researchers to observe the fluctuation of the phase boundaries driven by movement of the atoms from one phase to another, providing detail on how fast the oxygen vacancies filled and emptied during the transitions.
The researchers say that this new ability to see what is happening with the atoms in materials will enable novel studies of other complex oxides, such as those used in lithium batteries and fuel cells. ― Michael Allen
[1] “Origin of metal-insulator transitions in correlated perovskite metals”, M. Chandler Bennett, Guoxiang Hu, Guangming Wang, Olle Heinonen, Paul R. C. Kent, Jaron T. Krogel, and P. Ganesh., Phys. Rev. Research 4, L022005 (2022). DOI: 10.1103/PhysRevResearch.4.L022005
See: Qingteng Zhang1*, Guoxiang Hu2,3*, Vitalii Starchenko5*, Gang Wan1, Eric M. Dufresne1, Yongqi Dong1, Huajun Liu4, Hua Zhou1, Hyoungjeen Jeen5, Kayahan Saritas5, Jaron T. Krogel5, Fernando A. Reboredo5, Ho Nyung Lee5, Alec R. Sandy1, Irene Calvo Almazan1, Panchapakesan Ganesh3*, and Dillon D. Fong1**, “Phase transition dynamics in a complex oxide heterostructure,” Phys. Rev. Lett., 129, 235701 (30 November 2022).
Author affiliations: 1Argonne National Laboratory, 2City University of New York, 3Center for Nanophase Materials Sciences Division, Oak Ridge National Laboratory, 4Institute of Materials Research and Engineering, 5Materials Science and Technology Division, Oak Ridge National Laboratory
Correspondence: * ganeshp@ornl.gov, ** fong@anl.gov
Work on the thin-film sample was supported by U.S. Department of Energy (DOE) Office of Science-Basic Energy Sciences (BES), Materials Sciences and Engineering Division. V.S. was supported by the U.S. DOE Office of Science-BES, Chemical Sciences, Geosciences, and Biosciences Division. G. H., P. G. and J.T.K. were supported by the U.S. DOE Office of Science-BES, Materials Sciences and Engineering Division, as part of the Computational Materials Sciences Program at the Center for Predictive Simulation of Functional Materials. QMC calculations by K.S., J.T.K. and F.R. were supported by the U.S. DOE Office of Science-BES, Materials Sciences and Engineering Division. A portion of research was conducted at the Center for Nanophase Materials Sciences at Oak Ridge National Laboratory, which is a DOE Office of Science user facility. The QMC calculations used resources of the Oak Ridge Leadership Computing Facility at the Oak Ridge National Laboratory, which is supported by the U.S. DOE Office of Science under Contract No. DE-AC05-00OR22725. The DFT calculations used resources of the National Energy Research Scientific Computing Center (NERSC), a U.S. DOE Office of Science user facility located at Lawrence Berkeley National Laboratory, operated under Contract No. DE-AC02-05CH11231. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The Center for Nanophase Materials Sciences is one of the five DOE Nanoscale Science Research Centers, premier national user facilities for interdisciplinary research at the nanoscale supported by the DOE Office of Science. Together the NSRCs comprise a suite of complementary facilities that provide researchers with state-of-the-art capabilities to fabricate, process, characterize and model nanoscale materials, and constitute the largest infrastructure investment of the National Nanotechnology Initiative. The NSRCs are located at DOE’s Argonne, Brookhaven, Lawrence Berkeley, Oak Ridge, Sandia and Los Alamos National Laboratories. For more information about the DOE NSRCs, please visit http://science.energy.gov/bes/suf/user-facilities/nanoscale-science-res….
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
