The amazing response of the scientific community to the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic has led to the rapid development and approval of anti-SARS-CoV-2 vaccines, saving millions of lives. This has been followed by attempts to figure out what the virus will do next by tracking emerging variants of the initial strain and mapping changes in viral surface proteins that may help new coronavirus strains to evade immune detection. This immune evasion is normal behavior for viruses but all of us are eager to see the development of a vaccine that can protect against the various existing strains of coronavirus and, hopefully, against potential new strains as well, finally putting an end to this pandemic. Recent work conducted at the Advanced Photon Source (APS), a U.S. Department of Energy Office of Science user facility at Argonne National Laboratory, identified an antibody that recognizes and neutralizes a broad array of coronaviruses and used a structural approach to identify the viral epitope the antibody recognizes. This work, published in the journal Structure, provides the basis for the potential development of broadly effective anti-coronavirus vaccines and therapeutic antibodies.
The work was conducted by a collaborative team from the National Institutes for Health, Johns Hopkins University, and the Frederick National Laboratory for Cancer Research. The first step was to immunize mice with mRNA for the SARS-CoV-2 spike protein. The spike protein is important for latching onto host cells during infection and has been the target of successful vaccine-generation efforts. Injection of the spike protein mRNA into mice caused the mice to generate an antibody immune response to the viral protein. This allowed the researchers to collect these antibodies and amplify 11 of them into monoclonal antibodies that they could then characterize for their binding properties. One of the antibodies, called WS6, demonstrated broad and strong ability to bind to a diverse range of coronavirus strains in binding assays. WS6 was also able to neutralize infection in a cell-based assay using SARS-CoV-2 variants, including delta and omicron, and other related viruses, such as SARS-CoV-1, and coronaviruses isolated from bats, civets, and pangolins, species that may have transmitted these viruses into the human population.
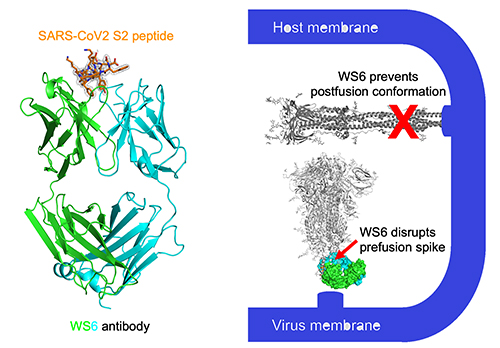
To understand how the WS6 antibody binding site is conserved among different coronaviruses and how the antibody blocks infection, the next step was to identify the location on the virus where WS6 was binding. The team tried negative stain electron microscopy to visualize the antibody binding to the virus but were unable to obtain a good snapshot of the binding event. Next, they turned to peptide-array epitope mapping and successfully identified a 17-residue sequence in the S2-domain of the SARS-CoV-2 spike protein where WS6 bound. By using this 17-amino acid peptide and the binding portion of the WS6 monoclonal antibody, the team solved the x-ray crystal structure of the complex to 2-Å resolution using the Southeast Regional Collaborative Access Team 22-ID x-ray beamline at the APS. The crystal structure showed that the peptide forms a three-turn helix and the antibody interacts with almost every residue of the helix except two that are oriented away from the antibody due to their location on the opposite face of the helix.
The structural information, cell-based assays, and further modeling of viral attachment also allowed the team to understand more about the neutralization ability of WS6. Comparison of antibodies with similar or different coronavirus neutralization profiles showed that those with different profiles appear to block neutralization before viral attachment whereas WS6 and related antibodies are able to block viral entry into cells after attachment by binding to a cryptic site on the spike protein that is revealed only upon antibody binding.
This binding site appears to represent a supersite of vulnerability that is conserved among a variety of coronaviruses and recognized by other effective neutralizing antibodies. This provides a unique site of vulnerability that could be used as a vaccine to focus the immune response to this supersite that is shared by different related coronaviruses and, perhaps, by new variants as they emerge. ― Sandy Field
See: Wei Shi1, Lingshu Wang1, Tongqing Zhou1, Mallika Sastry1, Eun Sung Yang1, Yi Zhang1, Man Chen1, Xuejun Chen1, Misook Choe1, Adrian Creanga1, Kwan Leung1, Adam S. Olia1, Amarendra Pegu1, Reda Rawi1, Arne Schön2, Chen-Hsiang Shen1, Erik-Stephane D. Stancofski1, Chloe Adrienna Talana1, I-Ting Teng1, Shuishu Wang1, Kizzmekia S. Corbett1, Yaroslav Tsybovsky3, John R. Mascola1*, and Peter D. Kwong1**, “Vaccine-elicited murine antibody WS6 neutralizes diverse beta-coronaviruses by recognizing a helical stemsupersite of vulnerability,” Structure 30, 1233 (2022). DOI: 10.1016/j.str.2022.06.004
Author affiliations: 1National Institutes of Health, 2Johns Hopkins University, 3Frederick National Laboratory for Cancer Research
Correspondence: * jmascola@icloud.com, ** pdkwong@nih.gov
Support for this work was provided by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health and by federal funds from the Frederick National Laboratory for Cancer Research under contract HHSN261200800001E. The Southeast Regional Collaborative Access Team is supported by its member institutions, and equipment grants (S10_RR25528, S10_RR028976, and S10_OD027000) from the National Institutes of Health. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
