Removing carbon dioxide from the atmosphere or from effluent from fossil fuel-burning entering the atmosphere is one way to limit climate change. One possible reaction to remove CO2 is to convert it to CO in an electrochemical carbon dioxide reduction reaction (CO2RR). In addition to using the CO as feedstocks for further conversion into liquid hydrocarbons, solar and wind energy can be stored in carbon-based fuels and chemicals using the electrochemical CO2 reduction reaction.
However, it remains a grand challenge for the CO2 to CO conversion at high selectivity (>90%) with large current densities. CO2 molecules are very stable; reduction requires a tremendous amount of energy and a catalyst. While precious metals (e.g., gold and silver) currently are the most efficient catalysts known for this reaction, costs make this process impractical on a large scale. The need for catalysts that are stable, abundant, and inexpensive is high, but until recently, identifying practical possibilities has been elusive.
For this paper, the researchers designed a highly efficient CO2 to CO reduction reaction using a high-performance, atomically dispersed and nitrogen (N) coordinated single Ni site catalyst embedded in partially graphitized carbon. Single Ni sites are highly efficient for CO2 reduction to CO. The researchers carefully controlled factors that had previously hindered this work, including carbon particle size, Ni content, and Ni-N bond structures and coordination. In addition, previously unknown extrinsic and intrinsic factors governing the performance of catalytic CO2 electrolysis and selectivity of single Ni site catalysts were studied experimentally and theoretically.
Energy-dispersive x-ray spectroscopy and the corresponding scanning transmission electron microscopy were conducted at the Center for Functional Nanomaterials at Brookhaven National Laboratory, which is a U.S. Department of Energy (DOE) Office of Science user facility. High-resolution scanning transmission electron microscopy and electron energy-loss spectroscopy were conducted at the Center for Nanophase Materials Sciences at at Oak Ridge National Laboratory, also a U.S. DOE Office of Science user facility. X-ray absorption spectroscopy measurements were performed at the APS X-ray Science Division Chemical & Materials Science Group’s beamline 12-BM at the APS, an Office of Science user facility at Argonne National Laboratory.
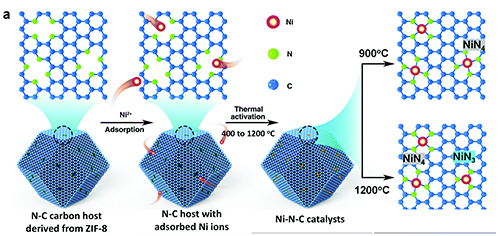
Compressively straining NiN4 sites could be used to shorten Ni-N bonds to enhance the CO2RR activity and the selectivity of the Ni-N-C catalyst (Fig. 1). However, NiN3 sites formed at higher temperature were more active and CO-selective than NiN4; this allowed researchers to design a catalyst using NiN3 sites with increased density. The team also studied how morphological factors, such as how carbon host particle size and Ni loading alter the final catalyst structure and performance.
The intensive engineering of the Ni-N-C catalyst included Ni-N bond structures, extrinsic particle size, and metal content. The implementation of this catalyst in an industrial flow-cell electrolyzer was impressive for CO generation with more than 90% efficiency. This turned out to be one of the best CO2 to CO catalysts, achieving a current density of CO up to 726 mA cm2 with faradaic efficiency of CO above 90%,
This research elucidates how thermal activation temperatures can affect the activity and selectivity of active sites in the CO2 reduction reaction. Advances in the field include a better understanding of the evolution of the atomically dispersed and nitrogen coordinated single Ni sites from small Ni-groupings during thermal activation, NiN4 moieties, and NiN3 behavior at high activation temperatures, including a lessening of N-coordination numbers and an increase in Ni-N bond strain. This new technology could generate CO from CO2 at an industrial scale using materials that are abundant and inexpensive. ― Dana Desonie
See: Yi Li1,2, Nadia Mohd Adli1, Weitao Shan3**, Maoyu Wang4, Michael J. Zachman5, Sooyeon Hwang6, Hassina Tabassum1, Stavros Karakalos7, Zhenxing Feng4***, Guofeng Wang3, Yuguang C. Li1****, and Gang Wu1*, “Atomically dispersed single Ni site catalysts for high-efficiency CO2 electroreduction at industrial-level current densities,” Energy Environ. Sci. 15, 2108 (2022). DOI: 10.1039/d2ee00318j
Author affiiations: 1State University of New York at Buffalo, 2Jiangsu University, 3University of Pittsburgh, 4Oregon State University, 5Oak Ridge National Laboratory, 6Brookhaven National Laboratory, 7University of South Carolina
Correspondence: * gangwu@buffalo.edu, ** guw8@pitt.edu, *** zhenxing.feng@oregonstate.edu, **** yuguangl@buffalo.edu
G. Wu and G. F. Wang acknowledge the support for a collaborative project from the U.S. National Science Foundation (NSF) (CBET-1804326 and 1804534). Z. Feng thanks the U.S. NSF (NNCI-2025489). Y. Li. thanks the Natural Science Foundation of Jiangsu Province (BK20210769). This research was conducted as part of a user project at the Center for Nanophase Materials Sciences, which is a US Department of Energy, Office of Science User Facility at Oak Ridge National Laboratory. This research used resources of the Center for Functional Nanomaterials (CFN), which is a U.S. Department of Energy Office of Science User Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
