Researchers at the University of California, Irvine, (UCI) and four U.S. Department of Energy (DOE) Office of Science national laboratories using three DOE x-ray light source user facilities, including the Advanced Photon Source (APS), have devised a way to make lithium-ion battery cathodes without using cobalt, a mineral plagued by price volatility and geopolitical complications.
In a paper published today in the journal Nature, the scientists describe how they overcame thermal and chemical-mechanical instabilities of cathodes composed substantially of nickel – a common substitute for cobalt – by mixing in several other metallic elements.
“Through a technique we refer to as ‘high-entropy doping,’ we were able to successfully fabricate a cobalt-free layered cathode with extremely high heat tolerance and stability over repeated charge and discharge cycles,” said corresponding author Huolin Xin, UCI professor of physics & astronomy. “This achievement resolves long-standing safety and stability concerns around high-nickel battery materials, paving the way for broad-based commercial applications.”
Cobalt is one of the most significant supply chain risks threatening widespread adoption of electric cars, trucks and other electronic devices requiring batteries, according to the paper’s authors. The mineral, which is chemically suited for the purpose of stabilizing lithium-ion battery cathodes, is mined almost exclusively in the Democratic Republic of Congo under abusive and inhumane conditions.
“Electric vehicle manufacturers are eager to curtail the use of cobalt in their battery packs not only for cost reduction but to counter the child labor practices used to mine the mineral,” Xin said. “Research has also shown that cobalt can lead to oxygen release at high voltage, causing damage to lithium-ion batteries. All of this points to a need for alternatives.”
However, nickel-based cathodes come with their own problems, such as poor heat tolerance, which can lead to oxidization of battery materials, thermal runaway and even explosion. Although high-nickel cathodes accommodate larger capacities, volume strain from repeated expansion and contraction can result in poor stability and safety concerns.
The researchers sought to address these issues through compositionally complex high-entropy doping using HE-LMNO, an amalgamation of transition metals magnesium, titanium, manganese, molybdenum and niobium in the structure’s interior, with a subset of these minerals used on its surface and interface with other battery materials.
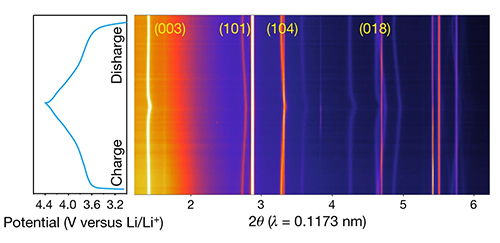
Xin and his colleagues employed an array of synchrotron x-ray diffraction, transmission electron microscopy and 3-D nanotomography instruments to determine that their zero-cobalt cathode exhibited an unprecedented volumetric change of zero during repeated use. The highly stable structure is capable of withstanding more than 1,000 cycles and high temperatures, which makes it comparable to cathodes with much lower nickel content.
The in situ heating and charge–discharge x-ray diffraction (XRD) and ex situ XRD (Fig.1) were performed on the X-ray Science Division Structural Science Group’s beamline 11-ID-C at the Advanced Photon Source at Argonne National Laboratory. The beamline is optimized for rapid high-energy XRD at 106 keV. Hard x-ray absorption spectroscopy of transition metal ions was performed on the QAS beamline 7-BM at the DOE’s National Synchrotron Light Source II (NSLS II) at Brookhaven National Laboratory. X-ray fluorescence mapping was performed at the 5-ID SRX beamline at NSLS II. TXM experiments were conducted at beamline 18-ID FXI at NSLS II, which offers advanced capabilities for studying the morphology and oxidation states of dynamic systems in two and three dimensions with 30-nm resolution. Soft x-ray absorption including the total electron yield and fluorescence yield modes was performed at beamline 10-1 at the Stanford Synchrotron Radiation Lightsource at the SLAC National Accelerator Laboratory.
“The combination of the different methods at the DOE light sources including the NSLS II beamlines enabled the discovery of a trapping effect of oxygen vacancies and defects inside the material, which effectively prevents the crack formation in the HE-LMNO secondary particle, making this structure extremely stable during cycling,” said co-author Mingyuan Ge, a scientist at NSLS-II.
Added Xin: “Using these advanced tools, we were able to observe the dramatically increased thermal stability and zero-volumetric-change characteristics of the cathode, and we’ve been able to demonstrate extraordinarily improved capacity retention and cycle life. This research could set the stage for the development of an energy-dense alternative to existing batteries.”
He said the work represents a step toward achieving the dual goal of spurring the proliferation of clean transportation and energy storage while addressing environmental justice issues around the extraction of minerals used in batteries.
See: Rui Zhang1, Chunyang Wang1, Peichao Zou1, Ruoqian Lin2, Lu Ma2, Liang Yin3, Tianyi Li3, Wenqian Xu3, Hao Jia4, Qiuyan Li4, Sami Sainio5, Kim Kisslinger2, Stephen E. Trask3, Steven N. Ehrlich2, Yang Yang2, Andrew M. Kiss2, Mingyuan Ge2, Bryant J. Polzin3, Sang Jun Lee5, Wu Xu4, Yang Ren3, and Huolin L. Xin1*, “Compositionally complex doping for zero-strain zero-cobalt layered cathodes,” Nature, published online 21 September 2022. DOI: 10.1038/s41586-022-05115-z
Author affiliations: 1University of California, Irvine, 2Brookhaven National Laboratory, 3Argonne National Laboratory, 4Pacific Northwest National Laboratory, 5SLAC National Accelerator Laboratory
Correspondence: * huolin.xin@uci.edu
This work is primarily supported by the U.S. Department of Energy (DOE) Office of Energy Efficiency and Renewable Energy (EERE) under award no. DE-EE0008444. The in situ TEM study of Li deintercalation is supported by the U.S. DOE Office of Sciences-Basic Energy Science, under award no. DE-SC0021204. The work done by R.Z. for this study was funded by the startup funding of H.L.X. R.L. was supported by the Assistant Secretary for EERE, Vehicle Technology Office of the U.S. DOE through the Advanced Battery Materials Research (BMR) Program, under contract no. DE-SC0012704. The work performed at Pacific Northwest National Laboratory was supported by the U.S. DOE Office of EERE through the Applied Battery Research Program under contract no. DE-AC05-76RL01830. This research used the resources of the Center for Functional Nanomaterials as well as the 7-BM, 18-ID, and 5-ID beamlines of the National Synchrotron Light Source II, which are two U.S. DOE Office of Science facilities at Brookhaven National Laboratory under contract no. DE-SC0012704. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. DOE Office of Science-Basic Energy Sciences under contract no. DE-AC02-76SF00515. The authors acknowledge the use of facilities and instrumentation at the UC Irvine Materials Research Institute, which is supported in part by the National Science Foundation through the UC Irvine Materials Research Science and Engineering Center (no. DMR-2011967). They also acknowledge the electrode produced at the U.S. DOE Cell Analysis, Modeling, and Prototyping (CAMP) Facility, Argonne National Laboratory. The CAMP Facility is fully supported by the DOE Vehicle Technologies Office. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
About the University of California, Irvine: Founded in 1965, UCI is a member of the prestigious Association of American Universities and is ranked among the nation’s top 10 public universities by U.S. News & World Report. The campus has produced five Nobel laureates and is known for its academic achievement, premier research, innovation and anteater mascot. Led by Chancellor Howard Gillman, UCI has more than 36,000 students and offers 224 degree programs. It’s located in one of the world’s safest and most economically vibrant communities and is Orange County’s second-largest employer, contributing $7 billion annually to the local economy and $8 billion statewide. For more on UCI, visit www.uci.edu.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
