Summer vacation in the Anthropocene is not the idealized cross-country family trip filled with cooler evenings and fireflies. Gasoline prices are too high to road trip and the weather is too hot to stay out long. But these seemingly unrelated problems might have related solutions. Environmental chemists and engineers are exploring ways to update existing industrial chemical processes that involve both carbon dioxide and synthetic fuels. Recent research by international collaborators using the U.S. Department of Energy’s Advanced Photon Source (APS) and published in the journal Applied Catalysis B: Environmental demonstrates a new, low-pressure catalyst for a chemical reaction which recycles carbon dioxide and allows the recycling process to be tuned for particular output products, specifically differentiating between synthetic natural gas and synthetic fuels, improving the effectiveness of existing pathways for carbon dioxide recycling.
Recycling carbon dioxide can be done in many ways. In the direct methanation method, the carbon dioxide is converted to synthetic gas in a process that involves adding hydrogen atoms, a procedure called hydrogenation. In the method using the reverse water-gas shift reaction, the carbon dioxide is converted to carbon monoxide, which is sent downstream to a Fischer-Tropsch synthesis reactor where the carbon monoxide is hydrogenated to primarily linear hydrocarbons, and then additional chemical processing is carried out to produce synthetic fuel. In both methods, the result is carbon dioxide recycled into a re-usable synthetic hydrocarbon. Since CO2 conversion requires a catalyst, creating a catalyst that could be used to select one method over the other would be valuable for improving our ability to recycle carbon dioxide. If the catalyst promoted a reaction environment opposed to long-chain hydrocarbon growth, the output of the reaction could be synthetic gas. Alternatively, the catalyst could promote the reverse water-gas shift reaction to produce to produce carbon monoxide, which would then be converted to long-chain hydrocarbons using a downstream process called Fischer-Tropsch synthesis, for the production of synthetic fuels.
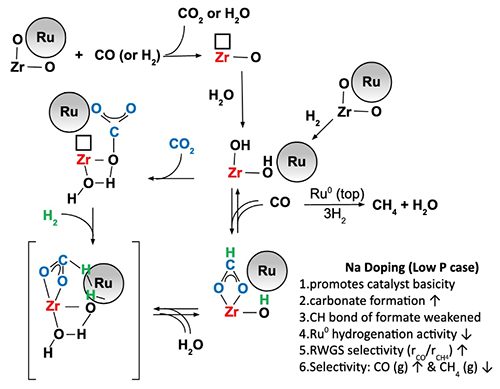
To explore possibilities for this tunable catalyst, the collaborators built on existing research. They utilized the efficacy of zirconia-supported metal nanoparticles at catalyzing the water-gas shift reaction. Additionally, they utilized the fact that substrates doped with sodium promote the water-gas shift reaction. By applying the chemical principle of microscopic reversibility, the team could incorporate substrates doped with sodium into their catalyst design that could also promote the reverse water-gas shift reaction. The team created a new catalyst incorporating ruthenium and sodium-doped zirconium dioxide. They varied the amount of sodium doping―none, 0.5%, 1.0%, 1.8%, 2.5%, and 5.0%―to allow them to measure the products generated during the recycling reactions as a function of the amount of sodium doping.
They performed extensive characterization of the catalysts before, during, and after using them to catalyze the hydrogenation reaction. The mechanism for the reaction is shown in Fig. 1. As part of the initial characterization, the team measured x-ray absorption near edge structure spectra and extended x-ray absorption fine structure spectra of the catalysts with the Materials Research Collaborative Access Team (MR-CAT) 10-ID-B beamline at the APS (the APS is an Office of Science user facility at Argonne National Laboratory). During the characterization, they also examined traits including the catalyst's response to temperature and the catalyst's surface composition. Following the hydrogenation reaction, they investigated the catalyst's stability.
The team found that increasing the sodium doping increased the basicity of the hydrogenation catalyst. The increased basicity of the reaction environment had two effects. One, it suppressed methanation: the sodium covered over ruthenium sites, suppressing their hydrogenation activity leading to methane. And second, the increased basicity promoted the reverse water-gas shift reaction: it accelerated the formation of formate and promoted the formation of carbon monoxide at the ruthenium-substrate interface. However, the higher amounts of sodium also negatively affected the catalyst's stability.
The team concluded that increasing the amount of sodium-doping assists the production of carbon monoxide (and the reverse water-gas shift reaction) and suppresses methanation. Thus, choosing specific amounts of sodium with which to dope the ruthenium and zirconium dioxide catalyst will allow environmental chemists to tune the reaction to promote downstream production of either synthetic gas (low sodium) or CO for the production of synthetic fuel (higher sodium), improving the effectiveness of existing pathways for carbon dioxide recycling. ― Mary Alexandra Agner
See: Raimundo C. Rabelo-Neto1, Mayra P. Almeida1,2, Erika B. Silveira1, Martin Ayala3, Caleb D. Watson3, Jesus Villarreal3, Donald C. Cronauer4, A. Jeremy Kropf4, Michela Martinelli5, Fabio B. Noronha1,2,6, Gary Jacobs3*, “CO2 hydrogenation: Selectivity control of CO versus CH4 achieved using Na doping over Ru/m-ZrO2 at low pressure,” Appl. Catal. B-Envir 315 121533 (2022). DOI: 10.1016/j.apcatb.2022.121533
Author affiliations: 1Instituto Nacional de Tecnologia, 2Instituto Militar de Engenharia, 3University of Texas at San Antonio, 4Argonne National Laboratory, 5 University of Kentucky Center for Applied Energy Research, 6 University of Lille
Correspondence: * gary.jacobs@utsa.edu
Raimundo C. Rabelo-Neto, Erika B. Silveira, and Mayra P. Almeida acknowledge the scholarship received from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - 302469/2020-6) and Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES – finance code 001), respectively. Fabio B. Noronha thanks CNPq (308200/2014-7 and 303667/201-4), CAPES – COFECUB program (88881.142911/2017-01) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ – E26/202.840/2017; E-26/010.253/ 2016) for the financial support. The journal article authors thank the National System of Nanotechnology Laboratories (MCTI/SisNANO/INT-CENANO-CNPq (442604/2019-0) for financial support. This study was also supported by the French government through the Programme Investissement d′ Avenir (I-SITE ULNE / ANR-16-IDEX-0004 ULNE) managed by the Agence Nationale de la Recherche, CNRS, M´etropole Europ´een de Lille (MEL) and Region Hauts-de-France, UTSA for “CatBioInnov” project are also acknowledged. Argonne’s research was supported in part by the U.S. Department of Energy (DOE) Office of Fossil Energy, National Energy Technology Laboratory (NETL). MR-CAT operations are supported by the U.S. DOE and the MR-CAT member institutions. CAER research was supported by the Commonwealth of Kentucky. Martin Ayala would like to thank the USDA National Institute of Food and Agriculture, Interdisciplinary Hands-on Research Traineeship and Extension Experiential Learning in Bioenergy/Natural Resources/Economics/Rural project, U-GREAT (Under Graduate Research, Education And Training) program (2016- 67032-24984) for financial support. Caleb D. Watson would like to acknowledge financial support from the Undergraduate National Science Foundation Research Program, supported by the National Science Foundation through grant award #1832388. Gary Jacobs would like to thank UTSA and the State of Texas for financial support through startup funds, as well as the Vice President of Research (UTSA) for an internal seed grant (UTSA-SWRI Connect). This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
