Lanthanides are a class of rare-earth metals perhaps best known for being part of the sets of chemical elements that are “out of order” on the periodic table. Though less familiar than other elements, lanthanides are used to produce a wide range of industrial and digital products, including magnets, batteries, lasers, and capacitors. As these products become more and more integrated in our daily lives, the demand for lanthanides has increased much faster than their supply. Surprisingly, though they are called rare-earth metals, lanthanides are relatively common in the Earth’s crust. What makes them rare is how difficult it is to extract them from rocks and then separate the different types of lanthanides from each other. The usual technique is to dissolve lanthanide-containing rocks in acids and then mix the solution with oil and surfactant until the lanthanide ions are transferred to the oil. However, this process is not very environmentally friendly and has to be repeated many times to approach industrial-level purity. To make this process more efficient, a team of researchers from Northwestern University employed a variety of synchrotron x-ray techniques at the U.S. Department of Energy’s Advanced Photon Source (APS) to learn how and why lanthanides collect at the water’s surface by taking a closer look at what types of lanthanide ions form surface layers and under what conditions. Their research, published in the journal Applied Materials & Interfaces, has the potential to help make lanthanide extraction more efficient and environmentally friendly.
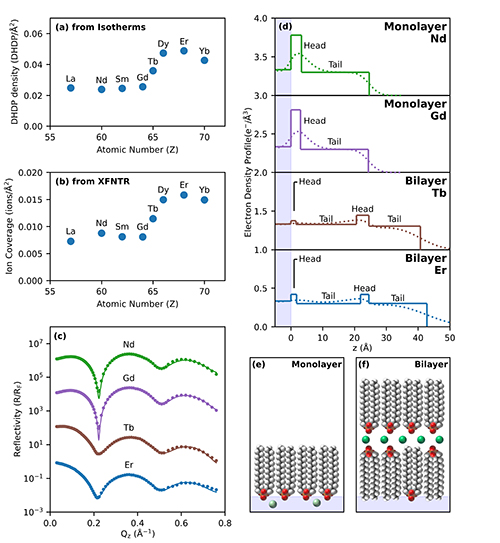
Previous research showed that lanthanide extraction can be made more efficient by the creation of a double layer of surfactant in a lanthanide mixture. Exactly why and how a bilayer formation occurs, and how it is related to the surfactant, is less clear. To solve this problem, researchers conducted x-ray experiments with lanthanide solutions at the ChemMatCARS 15-ID x-ray beamline of the APS at Argonne National Laboratory. The team spread dihexadecyl phosphate (DHDP), a common industrial surfactant, over a lanthanide-water solution at a variety of densities. They then compressed the solution to controlled amounts of total area and surface pressure and used an x-ray fluorescence technique to measure the surface concentration of fluorescing lanthanide ions. The technique works by directing x-rays at the surface of the solution at a very small angle such that only the surface is illuminated, allowing for the lanthanide ions to be detected and measured. X-ray reflectivity was used to determine the electron density profiles normal to the interface, and grazing incidence x-ray diffraction to determine the in-plane structure and ordering of ions and lipids at the interface.
Their results show that the lighter lanthanides, like lanthanum (La), neodymium (Nd), and samarium (Sm), react in similar and predictable ways, and that their presence is not correlated with bilayer formation. However, when the heavier lanthanides like erbium (Er) and dysprosium (Dy) are included along with additional concentrations of DHDP, an additional step can occur in which the DHDP monolayer “collapses” to form a bilayer structure. When this bilayer structure appears, DHDP density increases, and the heavier lanthanide ions tend to cluster in lines in a “sandwiched” region between a water-contacting layer and an air-contacting layer (Fig. 1). The result is that the heavier lanthanide ions become effectively isolated and the lanthanide ions become laterally ordered.
This research suggests that the efficiency of extraction processes can be enhanced by ensuring the conditions of a bilayer formation of surfactant that separates the heavier lanthanides. Once separated, it potentially becomes much easier to more efficiently transform the heavier lanthanides into their desired industrial form. ― Alicia Surrao
See: Sangjun Yoo, Baofu Qiao, Travis Douglas, Wei Bu, Monica Olvera de la Cruz, and Pulak Dutta*, “Specific Ion Effects in Lanthanide−Amphiphile Structures at the Air−Water Interface and Their Implications for Selective Separation,” ACS Appl. Mater. Interfaces 14, 7504 (2022). DOI: 10.1021/acsami.1c24008
Author affiliation: Northwestern University
Correspondence: * pdutta@northwestern.edu
This research was supported by the National Science Foundation (NSF) under grant number DMR-2004557. B.Q. and M.O.d.l.C. were supported by the grant DE-FG02-08ER46539 funded by the U.S. Department of Energy (DOE) Office of Science. ChemMatCARS Sector 15 is supported by the NSF under grant number NSF/CHE-1834750. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02- 06CH11357.
The U.S. Department of Energy's APS at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
