Nanoparticle self-assembly is a contemporary field that shows considerable promise in various sectors, including electronics and medicine. By definition, a nanoparticle has a size in the range of 1 to 100 nanometers. Because of their small size, nanoscale particles can adopt unique properties that make them well-suited for a particular application. One potential application is the construction of liquid surfaces with tunable properties. In order for self-assembly and surface ordering to occur, ligands (ions or molecules attached to a metal atom by coordinate bonding) must be provided to coat the surface of a nanoparticle core. Presently, very little is known about how ligands organize around a core and influence self-assembly. To learn more about this exciting field, researchers investigated how sulfur-containing thiol ligands interact with monolayers of water-supported gold-core nanoparticles. Using high-brightness x-rays at the U.S. Department of Energy’s (DOE’s) Advanced Photon Source (APS) as well as molecular dynamics simulations, the research team found that free thiols coat the core in a near-symmetrical fashion. These results, published in the journal Nano Letters, empower our collective understanding of nanoparticle self-assembly and may significantly impact how such assemblies are prepared for important societal applications.
In contrast to their larger material counterparts, nanoparticles possess a large ratio of surface area to volume. This enables them to harness desirable physical and chemical properties, which makes them quite appealing for a variety of industries, including drug delivery, imaging, and electronics. For example, nanoparticle-based drug delivery is appealing because nanoparticles can be designed to exhibit a high stability, have a large carrying capacity, can be conducive to different routes of administration, and possess specificity for target sites. Much research is currently under way in the sub-fields of nanomedicine and nano-delivery systems to treat cancer and enhance vaccine delivery. The recent FDA-approved vaccine for SARS-CoV-2 generated by Pfizer, for example, utilizes lipid nanoparticles.
One potential application of nanoparticles is the generation of novel liquid surfaces with tunable properties. For this to occur, ligands must be provided to coat the surface of a nanoparticle’s core. This coating impacts how a nanoparticle interfaces with a liquid surface and dictates how a nanoparticle self-assembles and orders itself. Historically, most research in the field has worked on designing ligands to induce a specific type of interaction or to encourage assembly toward a certain higher-order structure. In contrast, very little work has been done to understand how ligands organize on a core and how this organization impacts nanoparticle self-assembly and interactions.
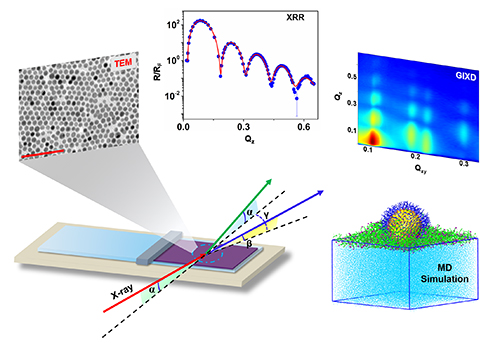
The current research has helped to overcome this gap in knowledge. The researchers chose to study thiol ligands, which are organosulfur compounds containing the chemical group R-SH, in conjunction with monolayers of gold-core nanoparticles supported by water. They employed x-ray scattering at the ChemMatCARS 15-ID x-ray beamline at the APS together with molecular dynamics simulations to investigate how these thiol ligands interact with and affect the metallic core of these nanoparticles (Fig. 1). (The APS is a DOE Office of Science user facility at Argonne National Laboratory.) The team discovered that free thiols thoroughly coat the gold core in a near-symmetrical fashion. Once core-ligand coverage exceeds a threshold value, the nanoparticle’s core rises above the water surface. The edge-to-edge distance increases in value, which means that the nanoparticles become more spaced apart. Consequently, the total nanoparticle coverage of the liquid surface is diminished.
In summary, this work demonstrates that free thiols not only regulate ligand organization on a nanoparticle's core, but they also influence how a nanoparticle interacts with its surroundings. By filling a key knowledge gap in the field of nanoparticle self-assembly, this essential research enhances our collective ability to construct nanoparticle materials. Given the relevance of nanoparticles to medicine, electronics, and other important industries, these discoveries could be flexibly utilized to help improve various aspects of society.
See: Pan Sun, Linsey M. Nowack, Wei Bu, Mrinal K. Bera, Sean Griesemer, Morgan Reik, Joshua Portner, Stuart A. Rice, Mark L. Schlossman, and Binhua Lin*, “Free Thiols Regulate the Interactions and Self-Assembly of Thiol-Passivated Metal Nanoparticles,” Nano Lett. 21(4), 1613 (2021). DOI: 10.1021/acs.nanolett.0c04147
Author affiliation: The University of Chicago
Correspondence: * lin@cars.uchicago.edu
This research is supported by ChemMatCARS. ChemMatCARS is funded by the Divisions of Chemistry (CHE) and Materials Research (DMR), National Science Foundation, under grant number NSF/CHE-1834750. We also acknowledge support from The University of Chicago MRSEC NSF/DMR-1420709 for B.L. and S.A.R., and NSF/ DMR-2011854 for J.P. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
