Research carried out at the U.S. Department of Energy’s Advanced Photon Source (APS) determined the distribution, speciation, and deleterious effects of antimony (Sb) and other toxic contaminants on the soil and the indigenous microbial community at an antimony refinery in South Korea. Researchers can now use this information to determine new and more effective ways to clean up antimony-contaminated sites. The results were published in the Journal of Hazardous Materials.
Antimony is a trace element in Earth’s crust, water, and soils. Although Sb is toxic, its alloys are used in batteries, printing presses, and bullets. Other antimony compounds appear in flame-retardant materials, paints, enamels, glass, and pottery. To isolate Sb for use, Sb-containing compounds must undergo mining and smelting.
Waste materials from ore refining are high in Sb and other toxic co-contaminants. Sb is genotoxic and so can damage DNA. Humans exposed to high levels of Sb may develop serious metabolic disorders. Sb also has deleterious effects on indigenous microorganisms. Sb methylation by microorganisms impacts Sb transformation and is critical in its biogeochemical cycle. Despite these dangers, South Korea does not limit antimony pollution, although many other nations, including the European Union, do.
To determine the best in situ and ex situ remediation strategies, researchers must understand the element’s speciation. The majority of Sb found in soils is as Sb(V), the mineral tripuhyite (FeSbO4), an immobile phase with low Sb bioavailability. Oxidation is caused by both abiotic and biotic processes. Little is known about the effects of co-contaminants, such as arsenic (As) and lead (Pb), on Sb, although they appear to help control Sb bioavailability and transport. For example, Sb(III) mobility is significantly decreased by adsorption to iron (hydr)oxides or by precipitation as Sb(III) sulfide.
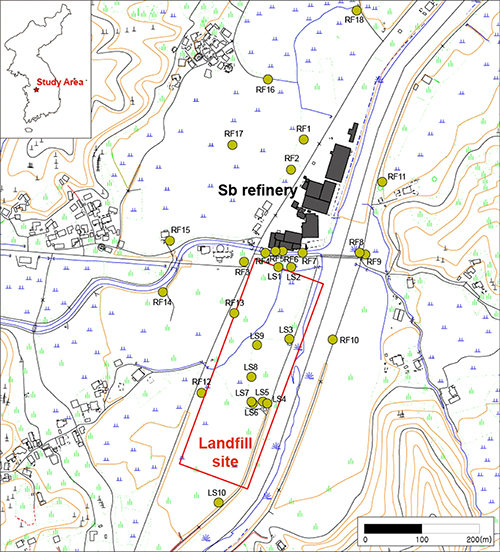
This study analyzed Sb and other toxic metal(loid)s near an operating Sb refinery and landfill site in South Korea (Fig. 1). In the 1980s, Sb-containing wastes were buried near the processing plant, resulting in local contamination. The site was remediated in the early 2000s by excavating and relocating the wastes. Nonetheless, there is still heavy residual contamination of Sb and the other toxic metal(oids) in the soil.
The researchers used antimony K-edge (30,491 eV) x-ray absorption near-edge spectroscopy (XANES) and extended x-ray absorption fine-structure (EXAFS) spectra collected under ambient conditions at the Materials Research Collaborative Access Team 10-BM x-ray beamline at the APS (the APS is an Office of Science user facility at Argonne National Laboratory). For microbial analyses, DNA was isolated, quantified, sequenced, and compared with DNA data from Sb-oxidizing bacteria.
In sediment samples taken horizontally and vertically, at both the refinery and landfill, Sb concentrations were high relative to EU guidelines. In the topsoil near the refinery, Sb levels were high, but other toxic metal(oids) were low. Some of the Sb appeared as Sb(III) in Sb2O3, but most was oxidized to Sb(V). Tripuhyite is stable with low solubility and so is a sink for Sb in oxidizing and supergene environments. At greater depths, concentrations of As and Pb increased; Sb was up to 15 times higher than in the topsoil. Sb concentrations decreased with distance from the refinery in the path of the prevailing wind.
Indigenous microorganisms are affected by Sb speciation and also by co-contamination with other heavy metal(oids). Throughout the study site, microbial populations were low relative to bulk soil but seemed unaffected by Sb. The populations were lowest where levels of co-contaminants were highest. Yet, even small differences in co-contaminants greatly influenced the composition of the microbial communities.
The researchers suggest that high Sb in topsoil is due to the release of Sb-containing dust from the refinery. Higher concentrations of Sb and other toxic metal(loid)s with soil depth is likely due to impurities in the Sb2O3 starting material used in earlier (1980s) refining operations. Apparently, this material was not removed during remediation in the 2000s. ― Dana Desonie
See: Soo-Chan Park1, Maxim I. Boyanov2,3, Kenneth M. Kemner3, Edward J. O’Loughlin3, and Man Jae Kwon1*, “Distribution and speciation of Sb and toxic metal(loid)s near an antimony refinery and their effects on indigenous microorganisms,” J. Hazard. Mater. 403 (2021). 123625 DOI: 10.1016/j.jhazmat.2020.123625
Author affiliations: 1Korea University, 2 Bulgarian Academy of Sciences, 3Argonne National Laboratory
Correspondence: * manjaekwon@korea.ac.kr
The authors thank the Materials Research Collaborative Access Team beamline staff for assistance during data collection. M.I.B., K.M.K., and E.J.O. were supported in part by the Wetland Hydrobiogeochemistry Scientific Focus Area (SFA) at Argonne National Laboratory funded by the Environmental System Science Program, Office of Biological and Environmental Research, Office of Science, U.S. Department of Energy (DOE), under contract DE-AC02-06CH11357. Materials Research Collaborative Access Team operations are supported by DOE and the Materials Research Collaborative Access Team member institutions. This work was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1A2B6001660) and by the Korea Environmental Industry & Technology Institute (KEITI) through the Subsurface Environment Management Project, funded by Korea’s Ministry of Environment (grant number 2018002440002). This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's Advanced Photon Source at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
