The original University of California, Irvine press release can be read here.
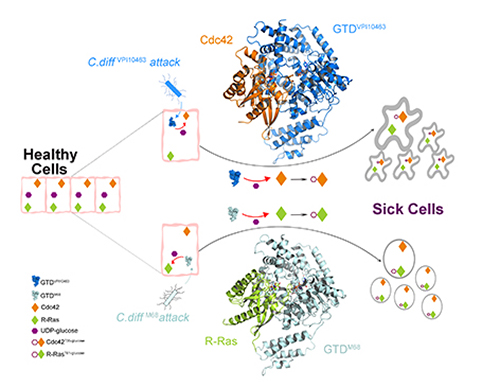
A University of California, Irvine (UCI)-led study suggests that the glucosyltransferase domain (GTD) is an ideal molecular target for therapeutic interventions for Clostridioides difficile infection (CDI). Based on their findings that established the structural basis for Toxin B recognition of the small GTPases Rho and R-Ras families, the study, which used data obtained at the U.S. Department of Energy’s (DOE’s) Advanced Photon Source and was published in Science Advances, may lead to new treatments to fight this deadly disease.
CDI is the leading cause of antibiotic-associated diarrhea and gastroenteritis-associated deaths worldwide, accounting for 500,000 cases and 29,000 deaths annually in the U.S. Classified by the Centers for Disease Control and Prevention as one of the top health threats, there is growing global concern surrounding the emerge and spread of hypervirulent C. difficile strains, resembling the occurrence of new virus variants in the current COVID pandemic. TcdB is one of two homologous C. difficile exotoxins, and TcdB alone is capable of causing the full spectrum of CDI diseases.
“We focused on the structure and function of TcdB’s crucial GTD, which is the toxin’s ‘warhead.’ The GTD is delivered by the toxin inside the host cells and causes most of the cytosolic damage to patients,” said Rongsheng Jin, professor in the Department of Physiology & Biophysics at the UCI School of Medicine, and corresponding author. “We discovered molecular mechanisms by which the GTD specifically recognizes and blocks the physiological functions of the human GTPases Rho and R-Ras enzyme families that are crucial signaling molecules.” To determine the structure of GTD, the team collected x-ray diffraction data at the Northeastern Collaborative Access Team (NE-CAT) beamline 24-ID-C of the Advanced Photon Source at Argonne National Laboratory.
The team also demonstrated how the classic form of TcdB and the hypervirulent TcdB recognize their human targets in different ways, which leads to distinct structural changes to the host cells caused by bacterial invasion.
“Once the GTD of TcdB is inside the cells, it is shielded by our cells and becomes inaccessible to passive immunotherapy. But our studies suggest that small molecule inhibitors could be developed to disarm the GTD, which will directly eliminate the root cause of disease symptoms and cellular damage,” Jin said. “This new strategy can potentially be integrated with and complement other CDI treatment regiments.”
See: Zheng Liu1, Sicai Zhang2, Peng Chen1, Songhai Tian2, Ji Zeng2, Kay Perry3, Min Dong2, and Rongsheng Jin1*, “Structural basis for selective modification of Rho and Ras GTPases by Clostridioides difficile toxin B,” Sci. Adv. 7, eabi4582 (22 October 2021). DOI: 10.1126/sciadv.abi4582
Author affiliations: 1University of California, Irvine, 2Harvard Medical School, 3Cornell University
Corresponding author: * r.jin@uci.edu
This work was partly supported by National Institutes of Health (NIH) grants R01AI125704, R21AI139690, and R21AI123920 to R.J.; R01NS080833 and R01AI132387 to M.D.; and R01AI139087 and R21 CA235533 to R.J. and M.D. M.D. holds the Investigator in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund. NE-CAT is supported by a grant from the National Institute of General Medical Sciences (P30 GM124165). This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's Advanced Photon Source at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
