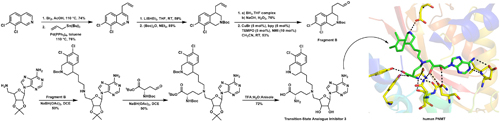
The enzyme phenylethanolamine N-methyltransferase (PNMT) is responsible for catalyzing the conversion of norepinephrine to epinephrine. It does this by transferring a methyl chemical group from a compound called S-adenosylmethionine (SAM) to norepinephrine. Evidence indicates that this protein may play a role in the age-related brain disorder Alzheimer's disease. In recent work published in the Journal of the American Chemical Society, a new series of synthetic PNMT inhibitors were described. These potent molecules are able to inhibit PNMT in the nanomolar range, and a structural analysis demonstrated how one of these inhibitors mediates this effect. Specifically, inhibitor 3 was shown to fill the catalytic binding pockets of SAM and the binding sites of norepinephrine, thereby preventing the ability of PNMT to work with either its cofactor (i.e., SAM) or its substrate (i.e., norepinephrine). The researchers in this study collected x-ray diffraction at the U.S. Department of Energy’s Advanced Photon Source (APS). Future research efforts are warranted to assess the effects of these new inhibitors in vivo and to determine whether or not they exacerbate disease symptoms in animal models of Alzheimer’s disease.
Alzheimer’s disease ranks as one of the most devastating age-related diseases. Most patients with Alzheimer’s disease get diagnosed over the age of 65 years and many suffer from severe symptoms including loss of ambulation, fecal and urinary incontinence, memory impairment, psychosis, and difficulty eating. The incidence of this disease grows every year and is projected to affect nearly 15 million Americans by 2050. There is a cogent need to better understand its pathogenesis and to design novel therapeutics capable of either delaying disease progression or reversing disease symptoms.
One interesting enzyme that may be relevant to Alzheimer’s disease is the methyltransferase enzyme phenylethanolamine N-methyltransferase (PNMT). PNMT converts norepinephrine/noradrenalin to epinephrine/adrenaline by transferring a methyl group to its substrate norepinephrine. This methyl group is obtained from a cofactor called S-adenosylmethionine (SAM). PNMT is expressed in the human brain and its activity is reportedly decreased in the brains of Alzheimer's disease patients. The activity of PNMT in the hippocampus—the part of the brain responsible for generating and storing long-term memories—negatively correlates with dementia severity. Put differently, a lower activity of PNMT in the hippocampus is associated with a more severe degree of dementia. Another laboratory reported that fear conditioning (a type of associative learning) is impaired in mice lacking the gene Pnmt. These data suggest that a reduction in PNMT may contribute to brain dysfunction.
In order to better study this enzyme, the researchers in this study from the Albert Einstein College of Medicine first solved the transition state structure of PNMT using isotope effects and computational chemistry, and published these results in ACS Chemical Biology. They designed, synthesized, and tested transition state analogues and then used the Lilly Research Laboratories Collaborative Access Team (LRL-CAT) 31-ID-D x-ray beamline at the APS to collect crystallographic data. These inhibitors were highly efficacious and are the first to be effective in a nanomolar range. The authors also performed an advanced structural analysis of one of these new molecules dubbed inhibitor 3. They showed that inhibitor 3 (Fig. 1) inhibits PNMT by filling the catalytic binding pockets of the cofactor SAM and the binding sites of the substrate norepinephrine. Key structural information was gleaned at LRL-CAT.
These inhibitors hold substantial promise as powerful research tools. For example, an experiment could be designed where aged animals are treated with a PNMT inhibitor. If treatment with this inhibitor leads to memory impairment or the development of symptoms that mimic dementia, it would strengthen the argument that PNMT plays an important role in Alzheimer’s disease. Alternatively, PNMT inhibitors could be administered in an animal model of Alzheimer’s disease to see if such inhibition exacerbates disease symptoms. It is also possible that there are other diseases where PNMT is overly active and this excess of activity is deleterious. If this is the case, then these inhibitors will play an immediate role as potential therapeutics.
Since the authors also learned more about PNMT, this new information could be used for the development of new drugs that enhance the activity of PNMT. Future studies are warranted to explore these possibilities. — Stephen Taylor
See: Niusha Mahmoodi, Rajesh K. Harijan, and Vern L. Schramm*, “Transition-State Analogues of Phenylethanolamine N‑Methyltransferase,” J. Am. Chem. Soc. 142, 14222 (2020). DOI: 10.1021/jacs.0c05446
Author affiliation: Albert Einstein College of Medicine
Correspondence: * vern.schramm@einsteinmed.org
This work was supported by National Institutes of Health (NIH) research grant GM041916. The Albert Einstein Crystallographic Core X-ray diffraction facility is supported by NIH Shared Instrumentation Grant S10 OD020068. Use of the LRL-CAT beamline was provided by Eli Lilly Co., which operates the facility. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
