Most of chemical and industrial manufacturing worldwide is driven by processes that involve heterogeneous catalysis, which is crucial for the large-scale production of all kinds of products and for countless other applications. This makes the quest for better, cheaper, and more versatile catalysts of enduring importance. In recent years, a new approach called single-atom catalysis (SAC) has offered fresh possibilities to increase catalyst selectivity and efficiency, but stability can be a problem. One possible solution is the use of ligand-coordinated supported catalysts (LCSCs). Researchers using the U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne National Laboratory demonstrated that the LCSC strategy both stabilizes the structure and activity of single-atom metal centers and also allows them to be tuned and engineered for specific catalytic applications. This work was the cover subject of the journal ChemSusChem.
The research team examined the structure and ethylene hydrogenation reactivity of three metal-organic platinum (Pt)-ligand complexes with different coordination motifs. They characterized the structure of each by a variety of techniques, including x-ray absorption spectroscopy (XAS) at the X-ray Science Division Spectroscopy Group’s beamline 9-BM at the APS (the APS is an Office of Science user facility at Argonne National Laboratory). In each catalyst, one of three ligands (PDO, DPTZ, or BPDCA) was coordinated to Pt atoms on a TiO2 surface.
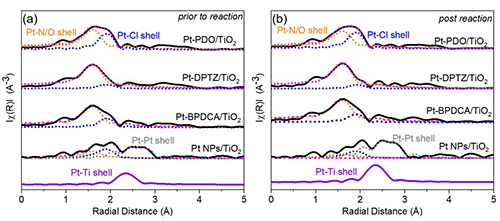
Extended x-ray absorption fine structure (EXAFS) measurements (Fig. 1) of the local coordination environment around the Pt centers in each of the LCSCs shows good Pt-N/O and Pt-Cl coordination without significant evidence of Pt scattering, demonstrating that most Pt on the catalyst surface is single-atom in nature without notable nanoparticle clustering. The coordination number of the Pt centers appears to be dependent on the choice of ligand.
Definite differences among the three ligands were seen in the ethylene hydrogenation reaction. The highest reaction rate, lowest reaction temperature and greatest stability were shown by LCSCs made with the PDO ligand, which achieved full conversion of ethylene to ethane at 30˚C. The BPDCA and DPTZ Pt-ligands required higher temperatures from 70˚C to as high as 110˚C. The latter two ligands also showed slower conversion rates.
The researchers attribute these differences among the three LCSC ligands to a variety of factors, including the Pt coordination number, which is lowest with the PDO ligand. This provides a greater number of active sites for Pt hydrogenation at lower temperatures, as shown by the EXAFS studies. Hydrogen dissociation is affected by temperature, and the Pt-ligands facilitate this at higher temperatures. The EXAFS experiments also show no Pt-Pt path throughout the reaction, which indicates the stability of the LCSC structures. The Pt ligands also appear to show no measurable changes in oxidation state or their coordination environment, also confirming their structural stability.
As a further check on the functioning of the LCSCs, the experimenters studied the Pt-TiO2 catalyst system without supporting ligands. They found that the absence of the ligands results in greater Pt clustering and aggregation under the same conditions, which indicates that these phenomena are suppressed when the ligand is present.
Finally, the team investigated the activity of the PDO LCSCs with iridium, another metal often considered for use in the hydrogenation catalysis process. The PDO ligand formed a stable and active coordination complex with iridium on TiO2. The hydrogenation reaction rate was notably slower with Ir than with Pt and followed a somewhat different reaction pathway, but was found to work well with no metal nanoparticle formation or clustering.
The experiments demonstrate the feasibility of ligand-supported coordinated catalysts for single-atom heterogeneous catalysis, showing their excellent stability and activity in the ethylene hydrogenation process under both ambient and reaction conditions. Of the three LCSCs tested, the Pt-PDO ligand proved the most favorable, indicating the importance of the choice of the particular ligand and its coordination with the single-atom metal centers. The work therefore suggests greater possibilities for custom-designing single-atom catalysts for specific purposes. ― Mark Wolverton
See: Xuemei Zhou1, 3, George E. Sterbinsky2, Eman Wasim1, Linxiao Chen1, 4, and Steven L. Tait1*, “Tuning Ligand-Coordinated Single Metal Atoms on TiO2 and their Dynamic Response during Hydrogenation Catalysis,” ChemSusChem 14(18). 3825 (September 20, 2021). DOI: 10.1002/cssc.202100208
Author affiliations: 1Indiana University, 2Argonne National Laboratory, 3Sichuan University, 4Pacific Northwest National Laboratory
Correspondence: * tait@indiana.edu
This work was supported by the U. S. Department of Energy (DOE) Office of Science-Basic Energy Sciences, Chemical Sciences program under Award Numbers DE-SC0016367 and DE-SC0021390. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
