Cartilage is a connective tissue made of solid and fluid components. Cartilage mechanics vary according to interactions between these components. Therefore, understanding how they interact is crucial to developing accurate models of how cartilage functions. In this study, researchers used high brightness x-rays at the U.S. Department of Energy’s Advanced Photon Source (APS), to directly measure solid/fluid interactions in a model system comprising bovine cartilage and various fluids. The qualities of the APS x-rays and the capacity of the technique they employed (x-ray photon correlation spectroscopy or XPCS) to capture nanometer-scale dynamics enabled the researchers to study these components at microscopic-length scales and depths for the first time, providing novel insight into factors that influence the mobility of the extracellular matrix (ECM) of cartilage. Their findings suggest that smaller ECM components are more mobile than larger components; dehydration slows mobility; and ECM dynamics are faster the closer they are to the cartilage surface. Their findings, published in the journal Osteoarthritis and Cartilage, also show that XPCS can be used to effectively measure ECM dynamics simultaneously at the submicron scale and the nanometer scale due to the use of large area detectors. Taken together, these results advance our understanding of cartilage dynamics and demonstrate a valuable new research tool.
Biological tissues perform complex mechanical functions, but questions remain regarding how the dynamics of biosolid-biofluid interactions affect tissue mechanics. Current cartilage biomechanics models are largely based on assumptions about how the viscous fluid interacts with the solid ECM, with many models showing that small shifts in nanoscale physical structures produce major effects. Direct measurements would help verify these models but capturing these interactions at the necessary nanoscale is challenging.
Small-angle x-ray photon correlation spectroscopy (SA-XPCS) can make these direct measurements at nanoscale. When a coherent x-ray beam from a light source like APS passes through a sample, the microstructure of the sample scatters the beam and generates an optical interference pattern called a “speckle” pattern on the x-ray detector. The motion of the sample microstructure creates time-dependent changes in the speckles, thereby conveying information about the sample’s dynamics with nanoscale sensitivity. Prior to this study, the only application of XPCS to biological samples was performed with complex fluids and protein solutions. In this study, half-moon-shaped cartilage samples (3 mm diameter and 3 mm thickness) from three juvenile bovine femoral condyles (two rounded prominences at the end of the femur) were inserted into a specimen holder and exposed to different conditions for specific XPCS experiments at the 8-ID-I beamline operated by the X-ray Science Division Dynamics & Structure Group at the APS.
First, a proof-of-concept experiment was performed showing hydration was necessary for XPCS to measure cartilage mobility. As cartilage contains multiple components of varying sizes and lengths, the researchers used XPCS to determine the lengths of components that contributed to ECM dynamics.
Cartilage has a hierarchical structure, such that the ECM composition and hydration varies at different depths into the sample. Thus, in the second experiment, the researchers assessed ECM mobility at various depths in cartilage exposed to bovine synovial fluid (the viscous liquid that bathes the cartilage in joints). XPCS was able to measure submicron- to nanometer-level fluctuations, making this the first study to show that biosolid-biofluid dynamics change with depth.
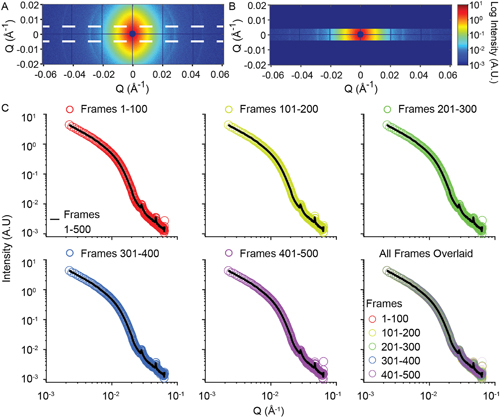
The cartilage ECM is often described as having different structural zones, all of which contain collage. Therefore, in the third experiment, the researchers evaluated the effects of collagen cross-linking to selectively determine collagen’s contribution to matrix dynamics. Collagen was cross-linked by soaking the cartilage samples in genipin and XPCS measurements were collected near the cartilage surface. They found that as cross-linking increases, ECM mobility decreases (Fig. 1).
Even though the surface of cartilage is in contact with synovial fluid, many experiments modeling ECM mobility use phosphate buffered saline or deionized water. In the fourth experiment, the researchers explored the effects of different fluids on ECM mobility. They exposed the cartilage samples to deionized water, phosphate buffered saline, or non-degraded bovine synovial fluid for 20 to 30 minutes, and then collected XPCS measurements near the cartilage surface. They found that ECM mobility was slowest in synovial fluid and fastest in deionized water.
In joint diseases like osteoarthritis (OA), the long hyaluronic chains in synovial fluid break down, causing a loss in synovial fluid viscosity. In the final experiment, the researchers evaluated whether a change in viscosity affected ECM mobility. They found that the less viscous the synovial fluid was, the more mobile the ECM became. This could affect the stability of the ECM at the surface of the cartilage as the disease advances.
This research is significant in a number of ways. First, the data collected at the APS can be used to expand our understanding of cartilage mobility and how it contributes to biomechanics of healthy and diseased joints. The data also help to verify the accuracy of assumption-based models, improve the design of cartilage therapies, and help scientists evaluate the effect that cartilage drugs have on the dynamics of the ECM. Finally, it shows the effectiveness of the XPCS technique in evaluating nanoscale dynamics in an opaque biological tissue and the spatial selectivity enabled by the micro-focused beam that allows for comparative studies at different regions of the sample. For these reasons, both the research itself and the XPCS that facilitated it may help scientists remediate joint diseases like osteoarthritis. ― Judy Myers
See: B.D. Partain1, Q. Zhang1, M. Unni1, J. Aldrich1, C.M. Rinaldi-Ramos1, S. Narayanan2, and K.D. Allen1*, “Spatially-resolved nanometer-scale measurement of cartilage extracellular matrix mobility,” Osteoarthr. Cartilage, 29(9), 1351 (September 2021). DOI: 10.1016/j.joca.2021.05.059
Author affiliations: 1University of Florida, 2Argonne National Laboratory
Correspondence: * kyle.allen@bme.ufl.edu
This work was supported by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases collection and analysis, manuscript preparation, or decision to publish. This work used resources from the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
