The original University of Chicago press release by Emily Ayshford can be read here.
A new University of Chicago study has found that the drug masitinib may be effective in treating COVID-19. The drug, which has undergone several clinical trials for human conditions but has not yet received approval to treat humans, inhibited the replication of SARS-CoV-2 in human cell cultures and in a mouse model, leading to much lower viral loads. Researchers at UChicago’s Pritzker School of Molecular Engineering (PME), working at the U.S. Department of Energy’s Advanced Photon Source (APS) with collaborators from Argonne National Laboratory and around the world, also found that the drug could be effective against many types of coronaviruses and picornaviruses. Because of the way it inhibits replication, it has also been shown to remain effective in the face of COVID-19 variants. These results were published in the journal Science.
“Inhibitors of the main protease of SARS-CoV-2, like masitinib, could be a new potential way to treat COVID patients, especially in early stages of the disease,” said Savas Tay, professor of molecular engineering at the PME, who led the research. “COVID-19 will likely be with us for many years, and novel coronaviruses will continue to arise. Finding existing drugs that have antiviral properties can be an essential part of treating these diseases.”
When COVID-19 lockdowns began in March 2020, Tay and article first author Nir Drayman, a PME postdoctoral fellow who specializes in virology, began to think about how they could help. To search for a better treatment for the disease, they began by screening a library of 1,900 clinically safe drugs against OC43, a coronavirus that causes the common cold and can be studied under regular biosafety conditions. They used cell cultures to determine the drugs’ effect on infection.
They then gave the top 30 drug candidates to microbiology professor and co-author Glenn Randall, who tested them in cell cultures against the SARS-CoV-2 virus at the Howard Taylor Ricketts Laboratory, a BSL-3 facility at Argonne. Measurements in the high-containment lab revealed nearly 20 drugs that inhibit SARS-CoV-2.
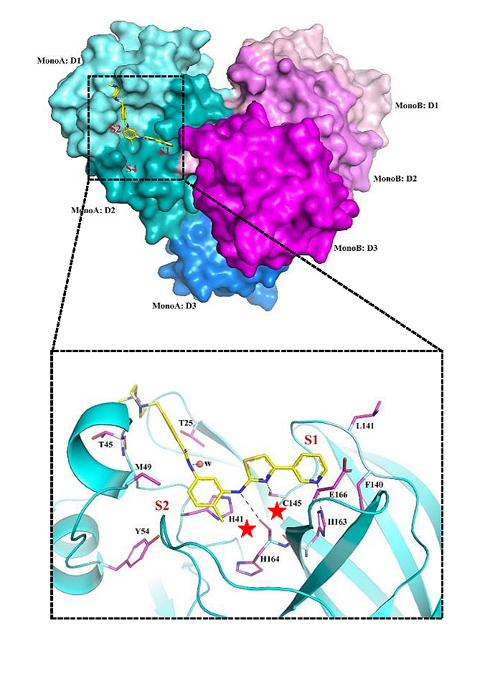
They also sent the drug candidates to other collaborators to test against the 3CL protease, the enzyme within coronaviruses that allows them to replicate inside a cell. They found that of the drug candidates, masitinib completely inhibited the 3CL viral enzyme inside the cell, a fact that was confirmed by x-ray crystallography at the X-ray Science Division Structural Biology Center (SBC-XSD) 19-ID x-ray beamline by Andrzej Joachimiak’s SBC-XSD group at the APS, an Office of Science user facility at Argonne. The drug specifically binds to the 3CL protease active site and inhibits further viral replication.
“That gave us a strong indication of how this drug works, and we became confident that it has a chance to work in humans,” Drayman said.
Though masitinib is currently only approved to treat mast cell tumors in dogs, it has undergone human clinical trials for several diseases, including melanoma, Alzheimer’s disease, multiple sclerosis, and asthma. It has been shown to be safe in humans but does cause side effects, including gastrointestinal disorders and edema, and could potentially raise a patient’s risk for heart disease.
Next, the researchers worked with peers at the University of Louisville to test the drug in a mouse model. They found that it reduced the SARS-CoV-2 viral load by more than 99 percent and reduced inflammatory cytokine levels in mice.
In parallel, the researchers also began to test the drug in cell cultures against other viruses and found that it was also effective against picornaviruses, which include Hepatitis A, polio, and rhinoviruses that cause the common cold.
They also tested it in cell cultures against three SARS-CoV-2 variants, Alpha, Beta, and Gamma, and found that it worked equally well against them, since it binds to the protease and not to the surface of the virus.
Now, the team is working with the pharmaceutical company that developed the drug (AB Science) to tweak the drug to make it an even more effective antiviral. Meanwhile, masitinib itself could be taken to human clinical trials in the future to test it as a COVID-19 treatment.
“Masitinib has the potential to be an effective antiviral now, especially when someone is first infected and the antiviral properties of the drug will have the biggest effect,” Drayman said. “This isn’t the first novel coronavirus outbreak, and it’s not going to be the last. In addition to vaccines, we need to have new treatments available to help those who have been infected.”
See: Nir Drayman1*, Jennifer K. DeMarco2, Krysten A. Jones1, Saara-Anne Azizi1, Heather M. Froggatt3, Kemin Tan1, 4, Natalia Ivanovna Maltseva1, 4, Siquan Chen1, Vlad Nicolaescu1, Steve Dvorkin1, Kevin Furlong1, Rahul S. Kathayat1, Mason R. Firpo5, Vincent Mastrodomenico5, Emily A. Bruce6, Madaline M. Schmidt6, Robert Jedrzejczak1, 4, Miguel Á. Muñoz-Alía7, Brooke Schuster1, Vishnu Nair1, Kyu-yeon Han8, Amornrat O’Brien5, Anastasia Tomatsidou1, Bjoern Meyer9, Marco Vignuzzi9, Dominique Missiakas1, Jason W. Botten6, Christopher B. Brooke10, Hyun Lee8, Susan C. Baker5, Bryan C. Mounce5, Nicholas S. Heaton3, William E. Severson2, Kenneth E. Palmer2, Bryan C. Dickinson1, Andrzej Joachimiak1, 4, Glenn Randall1, and Savaş Tay1*, “Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2,” Science, published online July 20, 2021. DOI: 10.1126/science.abg5827
Author affiliations: 1The University of Chicago, 2University of Louisville, 3Duke University, 4Argonne National Laboratory, 5Loyola University Chicago, 6University of Vermont, 7Mayo Clinic, 8University of Illinois at Chicago, 9Centre National de la Recherche Scientifique UMR 3569, 10University of Illinois at Urbana-Champaign
Correspondence: * tays@uchicago.edu, ** nirdra@uchicago.edu
The authors thank the members of SBC-XSD at Argonne National Laboratory, especially Darren Sherrell and Alex Lavens for their help with setting beamline and data collection at beamline 19-ID. N.D. is a recipient of the Human Frontiers Science Program (HFSP) post-doctoral fellowship. Funding for this project was provided in part by federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), Department of Health and Human Services, under Contract HHSN272201700060C (to A.J.) and by the U.S. Department of Energy (DOE) Office of Science through the National Virtual Biotechnology Laboratory, a consortium of DOE national laboratories focused on response to COVID-19, with funding provided by the Coronavirus CARES Act (to A.J.). E.A.B. acknowledges funding by NIH P20GM125498 (UVM Translational Global Infectious Disease Research Center) and NIH P30GM118228-04 (UVM Center for Immunology and Infectious Disease). J.W.B. acknowledges funding from the Office of the Vice President for Research at the University of Vermont and NIH grants R41AI132047, R21AI154198, and U01AI1141997. B.M. acknowledges the NIGMS grant R35GM138199. The use of SBC-XSD beamlines at the APS is supported by the U.S. DOE Office of Science and operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. B.D. acknowledges funding from the National Institute of General Medical Sciences (R35 GM119840). KP acknowledges funding from the NIH grant P20 GM125504. SCB acknowledges funding from NIH grant R01 AI085089. ST acknowledges funding support by the Pritzker School of Molecular Engineering at The University of Chicago. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357.
© 2021 The University of Chicago
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
