Trametinib, one example of a class of drugs known as MEK inhibitors, is a drug used to treat specific forms of skin and lung cancer and may be useful for treating several other forms of disease. MEK is an enzyme that controls a protein cascade that can malfunction in cancer, leading to the proliferation of cancer cells. While trametinib’s ability to stop MEK’s action makes it useful for treating some cancers, exactly how trametinib interacts with MEK was a mystery. A new study, which involved collecting x-ray diffraction data at the U.S. Department of Energy’s Advanced Photon Source (APS), solved this mystery by revealing the structure of trametinib and provides new insights into how it interacts with proteins involved in cancer. This study, published in the journal Nature, also describes the creation of a new compound called trametiglue, which works even better than trametinib at disrupting the cancer pathway. This discovery may pave the way for a new generation of cancer drugs.
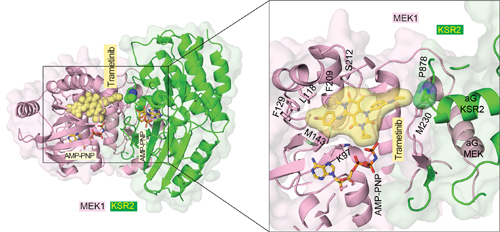
To determine the structural basis of the effects of trametinib on MEK, the researchers used macromolecular x-ray crystallography to study a crystal complex that included trametinib, MEK, and another protein known to interact with MEK called KSR. This allowed the researchers to deduce the three-dimensional structure of the molecular complex (Fig. 1). They also discovered something unexpected. Trametinib does not just bind to a pocket on MEK but also binds to the site where KSR interfaces with MEK. In addition, when KSR is bound to MEK it contorts MEK’s structure, including its binding pocket for trametinib.
The researchers then used other MEK inhibitors and found that trametinib was unique in its ability to bind to the interface between MEK and KSR. All in all, the researchers reported 12 new three-dimensional structures using the Life Sciences Collaborative Access Team (LS-CAT) 21-ID-F x-ray beamline of the APS at Argonne National Laboratory (Fig. 1), the National Synchrotron Light Source II beamlines 17-ID-1 AMX and 17-ID-2 FMX at Brookhaven National Laboratory, and beamline 8.2.2 of the Advanced Light Source at Lawrence Berkeley National Laboratory (all three are Office of Science user facilities).
Next, using an experiment with live cells in which a dye-conjugated form of trametinib participates in energy transfer reactions when bound to MEK, the researchers determined that the presence of KSR changes the amount of time that trametinib stays bound to MEK. They also found that trametinib disfavors MEK’s ability to bind to another protein called RAF. This finding might help explain why trametinib treatment can be less effective over time because free, non-sequestered RAF can create a workaround for the protein cascades that are mutated in cancer.
The new information about the structure of trametinib and how it interacts with other proteins involved in cancer provided the researchers with an exciting opportunity: they could engineer a new and better trametinib. They called their new compound “trametiglue.” Living up to its sticky name, trametiglue improves the interaction between MEK and KSR. Trametiglue has the added advantage of also promoting and stabilizing the interaction between MEK and RAF, essentially trapping RAF so it cannot become a force for drug resistance. Further good news came when Dar and colleagues tested trametiglue in a series of different types of cancer cells. They found that it was more effective at killing cancer cells at a lower dose than trametinib and other MEK inhibitors.
Trametiglue may not only be more potent and more effective and less susceptible to initiating drug resistance than trametinib, it may also pave the way for designing the next generation of drugs to treat a variety of cancers and other conditions. ― Summer Allen
See: Zaigham M. Khan, Alexander M. Real, William M. Marsiglia, Arthur Chow, Mary E. Duffy, Jayasudhan R. Yerabolu, Alex P. Scopton, and Arvin C. Dar*, “Structural basis for the action of the drug trametinib at KSR-bound MEK,” Nature 588, 509 (17 December 2020). DOI: 10.1038/s41586-020-2760-4
Author affiliation: Icahn School of Medicine at Mount Sinai
Correspondence: * arvin.dar@mssm.edu
We thank the staff at LS-CAT and the National Synchrotron Light Source II for help with x-ray diffraction experiments. The Dar laboratory has been supported by innovation awards from the NIH (1DP2CA186570-01) and Damon Runyan Rachleff Foundation, as well as National Institutes of Health (NIH) grants 1RO1CA227636 and 5U54OD020353. The authors are also supported by National Cancer Institute grant P30 CA196521 to the Tisch Cancer Institute. A.M.R. and W.M.M. are recipients of NIH F30 (CA232454) and F99/K00 (CA212474) awards, respectively. A.C. and M.E.D. are recipients of T32 fellowships 5T32CA078207 and 5T32GM062754, respectively. A.C.D. has been supported as a Pew-Stewart Scholar in Cancer Research and a Young Investigator of the Pershing-Square Sohn Cancer Research Alliance. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
