The original UCLA Samueli School of Engineering article can be read here.
Chemical engineers at the University of Illinois Chicago and UCLA used the U.S. Department of Energy’s Advanced Photon Source (APS) in answering longstanding questions about the underlying processes that determine the life cycle of liquid foams. The breakthrough in understanding how liquid foams dissipate could help improve the commercial production and application of foams in a broad range of industries and could lead to improved products. Findings of the research were featured in the Proceedings of the National Academy of Sciences of the United States of America.
Foams are a familiar phenomenon in everyday lives — mixing soaps and detergents into water when doing dishes, blowing bubbles out of soapy water toys, sipping the foam off a cup of lattes or milk shake. Liquid foams can occur in a variety of natural and artificial settings. While some foams are produced naturally, as in bodies of water creating large ocean blooms on the beaches, others arise in industrial processes. In oil recovery and fermentation, for example, foams are a byproduct.
Whenever soapy water is agitated, foams are formed. They are mostly gas pockets separated by thin liquid films that often contain tiny molecular aggregates called micelles. Oily dirt, for example, is washed away by hiding in the water-phobic cores of micelles. In addition, fat digestion in our bodies relies on the role of micelles formed by bile salts.
Over time, foams dissipate as liquid within the thin films is squeezed out. Soap and detergent molecules that are by very nature amphiphilic (hydrophilic and hydrophobic) aggregate within water to form spherical micelles, with their outward-facing heads being hydrophilic and water-phobic tails forming the core.
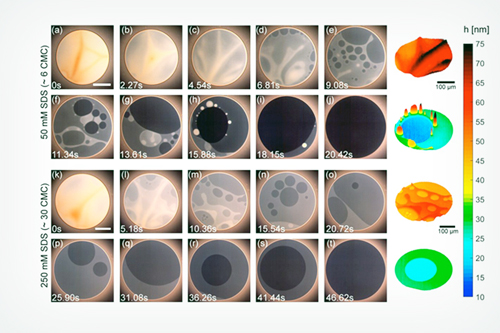
Micelles are tiny, but influential, not just in cleaning and solubilizing oil-loving molecules but also in affecting flows within foam films,” said co-principal investigator Vivek Sharma, an associate professor of chemical engineering at the UIC College of Engineering. For nearly a decade, he has pursued the question of how and why the presence of micelles leads to stepwise thinning, or stratification, within ultrathin foam films and soap bubbles.
To solve the puzzle, Sharma and his collaborators developed advanced imaging methods they call IDIOM (interferometry digital imaging optical microscopy) protocols that are implemented with high speed and digital single-lens reflex (DLSR) cameras. They found that foam films have a rich, ever-changing topography, and the thickness differences between different strata are much greater than the size of micelles.
“We used the precision technique called small-angle x-ray scattering [SAXS] to resolve the micelles’ shape, sizes, and densities,” said co-principal investigator Samanvaya Srivastava, an assistant professor of chemical and biomolecular engineering at the UCLA Samueli School of Engineering. “We found that the foam film thickness decreases in discrete jumps, with each jump corresponding to the exact distance between the micelles in the liquid film.” The SAXS studies were performed on the X-ray Science Division Chemical & Materials Science’s 12-ID-B x-ray beamline at the APS, a DOE Office of Science user facility at Argonne National Laboratory.
The team also discovered that the arrangement of micelles in foam films is governed primarily by the ionic interactions between micelles. The electrostatic attraction and repulsion between ions influences how long foams remain stable and how their structure decays. With these findings, the researchers determined that by simply measuring the foam film thickness, which can be accomplished with a DSLR camera using the IDIOM protocols, they could characterize both the nanoscale interactions of micelles in liquids and the stability of the foams.
Compared to previous techniques that are more time-consuming and require expensive, customized equipment, the new method is not only less expensive but is also more comprehensive and efficient.
“The knowledge and understanding could aid in the development of new products — from food and personal care to pharmaceuticals,” said the study’s co-lead authors, graduate students Shang Gao of UCLA Samueli and Chrystian Ochoa of UIC. “It could also help engineers improve the control of foams in industrial processes.”
See: Chrystian Ochoa1, Shang Gao2, Samanvaya Srivastava2*, and Vivek Sharma1**, “Foam film stratification studies probe intermicellar interactions,” Proc. Natl. Acad. Sci. 118(25), e2024805118 (2021). DOI: 10.1073/pnas.2024805118
Author affiliations: 1University of Illinois at Chicago, 2University of California, Los Angeles
Correspondence: * samsri@ucla.edu, ** viveks@uic.edu
V.S. acknowledges funding support from NSF-CBET 1806011 (National Science Foundation– Chemical, Bioengineering, Environmental and Transport Systems) and the UIC College of Engineering. S.G. and S.S. acknowledge funding support from the UCLA Samueli School of Engineering and the Department of Chemical and Biomolecular Engineering. We acknowledge the support provided by Dr. Xiaobing Zuo. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357.
© Copyright 2021 UCLA Samueli School Of Engineering
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
