Lithium-based batteries need improvement. Lithium-ion batteries are lightweight with high energy density, and therefore commonly used in phones, laptops, and electric vehicles. However, they have limited charge capacity, which is inherent to their structure and which restricts the range of electric cars and shortens how long we can talk on our phones before recharging. A newer type of battery, made of lithium metal instead of lithium ions, could hold much more charge. But first we need to solve key material challenges associated with these batteries. In particular, lithium metal batteries are prone to shorting and material damage when the lithium metal meets the ceramic parts of the battery. This can sharply limit the battery’s useful life. Now, a group of researchers has used the U.S. Department of Energy’s Advanced Photon Source (APS) to image the lithium-ceramic interface and explain why the damage occurs. Their research, which could lead to a new generation of much better batteries made of lithium metal, were published in Applied Energy Materials.
The standard lithium ion batteries we use in our devices today are made of a host material such as graphite, within which lithium ions are stored. This limits the amount of charge they can carry, because so much of the space and weight of the battery is taken by the host material. If lithium ion batteries are like water in a sponge, lithium metal batteries are like a block of ice. You can carry much more lithium—and thus much more energy—in the same space. A car using a lithium metal battery could drive three times the distance it could with a lithium ion battery of the same size.
But lithium metal has challenges. As the battery cycles through charging and discharging, the lithium has a tendency to grow dendrites, long roots of metal that worm into the ceramic sections of the battery and crack it. The lithium metal also has a tendency to peel away from the ceramic, breaking the contact and reducing the battery’s capacity. That in turn reduces the amount of time a device can run or drive without recharging.
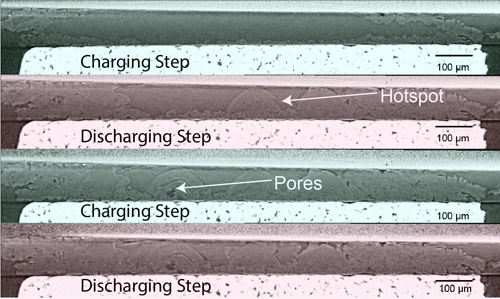
A team of researchers from Vanderbilt University with a colleague from Purdue University wanted to understand the physical reasons why lithium metal grows dendrites and cracks. Lithium metal batteries are challenging to image because lithium atoms are small and lightweight, so the absorption contrast between the metal and holes in the metal is very small. Fortunately, with assistance from scientists in the X-ray Science Division’s Materials Physics & Engineering Group, they were able to use the 1-ID-E beamline at the APS (an Office of Science user facility at Argonne National Laboratory) to accomplish this. The high flux of APS x-rays combined with a highly monochromatic beam (set to 72 keV) allowed the researchers to optimize the contrast so they could distinguish between lithium metal and empty voids.
The voids indeed revealed the source of the trouble. They create an imbalance of charge and electric flux. The imbalance stresses the material at the edges of the voids, and can lead to cracking, dendrite formation, or other degradation of the battery (Fig. 1). Researchers had guessed that this might be the case, but the research team’s images from the APS are the first experimental validation of this hypothesis.
Now that the researchers have confirmed the underlying physics of the problem, they have begun working on mitigating it. There are several strategies that could help, including coating the interface between the lithium metal and ceramic to allow for smoother metal deposition, reducing voids, and potentially changing the operating pressure of the battery.
The research is at the point where material scientists can begin work on actual, improved solid state lithium energy storage that could find practical use as the next generation of batteries in everyday devices. ― Kim Krieger
See: Marm B. Dixit1, Ankit Verma1, Wahid Zaman1, Xinlin Zhong1, Peter Kenesei2, Jun Sang Park2, Jonathan Almer2, Partha P. Mukherjee3, and Kelsey B. Hatzell1*, “Synchrotron Imaging of Pore Formation in Li Metal Solid-State Batteries Aided by Machine Learning,” ACS Appl. Energy Mater. 3, 9534 (2020). DOI: 10.1021/acsaem.0c02053
Author affiliations: 1Vanderbilt University, 2Argonne National Laboratory, 3Purdue University
Correspondence: *kelsey.b.hatzell@vanderbilt.edu
K.B.H. and M.B.D. acknowledge support from National Science Foundation (NSF) Grant 1847029. K.B.H. and W.Z. acknowledge support from NSF Grant 1821573. P.P.M. and A.V. acknowledge financial support in part from a Scialog program sponsored jointly by Research Corporation for Science Advancement and the Alfred P. Sloan Foundation, which includes a grant to Purdue University by the Alfred P. Sloan Foundation. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
