The original Oregon State University press release can be read here.
Lithium-ion batteries are critical for modern life, from powering our laptops and cell phones to those new holiday toys. But there is a safety risk – the batteries can catch fire. Zinc-based aqueous batteries avoid the fire hazard by using a water-based electrolyte instead of the conventional chemical solvent. However, uncontrolled dendrite growth limits their ability to provide the high performance and long life needed for practical applications. Now scientists who employed a number of research techniques including work at the U.S. Department of Energy’s Advanced Photon Source (APS) have reported in Nature Communications that a new three-dimensional (3-D) zinc-manganese nano-alloy anode has overcome the limitations, resulting in a stable, high-performance, dendrite-free aqueous battery using seawater as the electrolyte.
Yang Yang, corresponding author for the work and an assistant professor of Nanoscience Technology Center at the University of Central Florida (UCF), said the discovery offers promise for energy storage and other applications, including electric vehicles and electric grids.
“Aqueous electrolytes are cost-competitive, environmentally benign, capable of fast charging and high power densities, and highly tolerant of mishandling” he said. “Our zinc-based aqueous battery therefore is low-cost and stable, and has high energy density, which has the potential for large-scale manufacturing.”
Xiaonan Shan, co-corresponding author of the work and an assistant professor of Electrical and Computer Engineering at the University of Houston (UH), developed an in situ optical visualization technique, allowing the collaborative team to directly observe the reaction dynamics on the anode in real time. “This platform provides us with the capability to directly image the electrode reaction dynamics in situ,” Shan said. “This important information provides direct evidence and visualization of the reaction kinetics and helps us to understand phenomena that could not be easily accessed previously.”
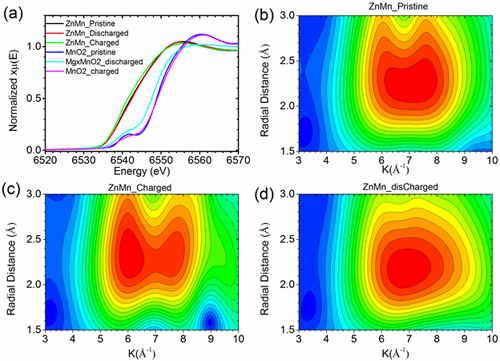
Testing determined that the novel 3-D zinc-manganese nano alloy anode remained stable without degrading throughout 1,000 hours of charge/discharge cycling under high current density (80 mA/cm2).
The anode is the electrode which releases current from a battery, while electrolytes are the medium through which the ionic charge flows between the cathode and anode. Using seawater as the electrolyte rather than highly purified water offers another avenue for lowering battery cost.
Traditional anode materials used in aqueous batteries have been prone to dendrites, tiny growths that can cause the battery to lose power. The team proposed and demonstrated a strategy to efficiently minimize and suppress dendrite formation in aqueous systems by controlling surface reaction thermodynamics with a zinc alloy and reaction kinetics by a three-dimensional structure.
To confirmed how the 3-D alloy was functioning in the battery, synchrotron x-ray characterizations play a vital role. Zhenxing Feng, co-corresponding author of the work and an assistant professor of Chemical Engineering at Oregon State University (OSU), together with the beamline scientist Hua Zhou at the APS, used x-ray absorption spectroscopy at the X-ray Science Division Chemical & Materials Science Group’s beamline 12-BM at the Advanced Photon Source to track the atomic and chemical changes of the anode in different operation stages. “Our theoretical and experimental studies proved that the 3D alloy anode has unprecedented interfacial stability, achieved by a favorable diffusion channel of zinc on the alloy surface,” Feng said. “The concept demonstrated in this collaborative work is likely to bring a paradigm shift in the design of high-performance alloy anodes for aqueous and non-aqueous batteries, revolutionizing the battery industry.”
Researchers at UCF, UH and OSU are currently investigating other metal alloys, in addition to the zinc-manganese alloy.
See: Huajun Tian1, Zhao Li1, Guangxia Feng2, Zhenzhong Yang3, David Fox1, Maoyu Wang4, Hua Zhou5, Lei Zhai1, Akihiro Kushima1, 6, Yingge Du3, Zhenxing Feng4*, Xiaonan Shan2**, and Yang Yang1, 6***, “Stable, high-performance, dendrite-free, seawater-based aqueous batteries,” Nat. Commun. 12, 237 (2021). DOI: 10.1038/s41467-020-20334-6
Author affiliations: 1University of Central Florida, 2University of Houston, 3Pacific Northwest National Laboratory, 4Oregon State University, 5Argonne National Laboratory, 6University of Central Florida
Correspondence: * zhenxing.feng@oregonstate.edu, ** xshan@central.uh.edu, *** Yang.Yang@ucf.edu
This work was primarily supported by the National Science Foundation under grant no. CBET-1949840, CMMI-1851674, CBET-1949870, CBET-2016192, and the startup grant from the University of Central Florida (UCF). H.T. thanks for the Preeminent Postdoctoral Program (P3) at UCF. Z.F. thanks for the startup funding from Oregon State University. TEM and data analysis were supported by the U.S. Department of Energy (DOE) Office of Science-Basic Energy Sciences, and Early Career Research Program under award #68278. A portion of the research was performed using EMSL, a DOE User Facility sponsored by the Office of Biological and Environmental Research and located at the Pacific Northwest National Laboratory. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02–06CH11357.
©2021 University of Houston. All rights reserved.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
