The original University of Michigan press release can read here.
Using data from experiments at the U.S. Department of Energy’s Advanced Photon Source (APS), researchers from the University of California, Berkeley, the University of Michigan, the Harvard Medical School, and the Harvard School of Public Health have discovered an antibody that blocks the ability of the dengue virus to cause disease in mice. The findings open the potential for developing effective treatments and designing a vaccine for dengue and similar diseases.
Dengue virus, a member of a group of viruses called flaviviruses, causes 50 to 100 million cases of dengue disease each year, with no effective treatment or vaccine. Other members of this group include the viruses that cause Zika, yellow fever and West Nile fever.
In a study published in the journal Science, the researchers revealed how an antibody called “2B7” neutralizes one specific protein made by the virus—a protein that is key to the dengue virus’s ability to both replicate and cause disease.
This protein, (NS1, short for “non-structural protein 1”) circulates in the patient’s blood and exacerbates disease by interacting directly with endothelial cells, the cells that form protective barriers around organs. By breaking apart the connections between endothelial cells, NS1 weakens this barrier, increasing permeability and contributing to increased vascular leak, which is the hallmark of severe dengue disease. This endothelial permeability may also enable the virus to more easily cross barriers to infect and damage target organs.
The authors and other researchers had previously demonstrated that this protein itself can cause leaks in the endothelial barrier, even in the absence of infectious viral particles. And in cases of dengue virus infection, the more NS1 found circulating in the host’s blood, the more severe the infection is likely to be.
“We think of bacterial toxins, but this idea of a viral toxin is a new concept,” said Eva Harris, a professor of infectious diseases and vaccinology at UC Berkeley’s School of Public Health and one of the study’s senior authors. “This is really an important protein in terms of creating new paradigms regarding how we think about viral proteins and their functions in disease.”
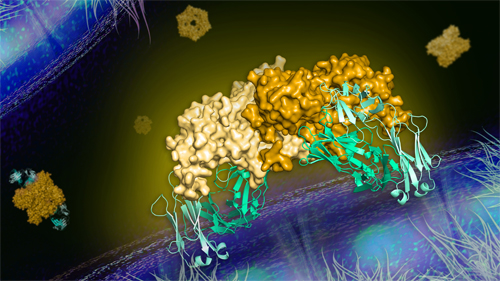
In this latest study, the researchers employed macromolecular crystallography and high-brightness x-rays at the National Institute of General Medical Sciences and National Cancer Institute (GM/CA-XSD) structural biology facility beamline 23-ID-B at the APS to identify specific regions of the protein that are responsible for damaging the endothelial cells: a so-called wing region that allows the protein to connect to the host cells, and another region that triggers destructive events within the endothelial cells. The APS is an Office of Science user facility at Argonne National Laboratory.
By analyzing the precise way that the 2B7 antibody attaches to the protein (Fig.1), they found that the antibody is able to neutralize both of these regions—simply by getting in the protein’s way. The antibody connects to NS1 in such a way that the wing regions cannot reach the endothelial cells, preventing the protein from latching onto (and thus interacting with and damaging) the endothelial cells.
“This collaborative approach gives us a lot of great insight into understanding the biology of this protein, its interactions with cells and its pathogenesis,” said David Akey, a researcher at the University of Michigan Life Sciences Institute and a lead author of the study. “It’s an example of combining structure and function to open therapeutic avenues.”
One reason no effective therapeutic has been found for dengue is that the disease can be caused by one of four different virus strains (dengue virus 1, 2, 3 or 4). Having antibodies against one strain of the virus can actually increase severity of a subsequent infection from another strain, a phenomenon called antibody-dependent enhancement.
By binding only to the NS1 protein and not to the virus particle itself, however, the 2B7 antibody does not lead to antibody-dependent enhancement of the infection.
“These findings tell us that we can really have an effect on the virus’s pathogenesis by blocking these sites on just the circulating proteins,” said Janet Smith, a professor at the University of Michigan Life Sciences Institute and the University of Michigan Medical School. “It offers a strategy not only for a therapy to treat an infection, but also for a vaccine to prevent infection.”
And because the NS1 protein is produced by many flaviviruses, the scientists believe the antibody that targets NS1 may be useful in treating or preventing multiple flaviviruses.
“We were able to show not only the mechanism of how the antibody protects the host cells, but also the actual mechanism of pathogenesis of this protein that is conserved across other flaviviruses,” Harris said.
“I think the fact that this antibody is cross-reactive with other flavivirus NS1 proteins is one of the most exciting elements of this work,” said Scott Biering, a postdoctoral researcher in Harris’s lab and a lead author of the study. “This research is the proof of concept that you can target this one protein for multiple flaviviruses to protect against pathogenesis. It opens a lot of avenues not only for better understanding the mechanics of this virus, but also for developing effective therapeutics.”
See: Scott B. Biering1, David L. Akey2, Marcus P. Wong1, W. Clay Brown2, Nicholas T.N. Lo1, Henry Puerta-Guardo1, Francielle Tramontini4, Gomes de Sousa1, Chunling Wang1, Jamie R. Konwerski2, Diego A. Espinosa1, Nicholas J. Bockhaus2,3, Dustin R. Glasner1, Jeffrey Li1, Sophie F. Blanc1, Evan Y. Juan1, Stephen J. Elledge4, Michael J. Mina5, P. Robert Beatty1, Janet L. Smith2,3*, Eva Harris1**, “Structural basis for antibody inhibition of flavivirus NS1-triggered endothelial dysfunction,” Science 08, published on line January 2021. DOI: 10.1126/science.abc0476.
Author affiliations: 1University of California, Berkeley, 2University of Michigan, 3University of Michigan Medical School, 4Harvard Medical School, 5Harvard School of Public Health
Correspondence: **eharris@berkeley.edu, *janetsmith@umich.edu
This work was supported by the National Institute of Allergy and Infectious Diseases National Institutes of Health (NIH) grants R01 AI24493 (E.H.), R21 AI146464 (E.H.), U19 AI109761 (E.H., Program Director I. Lipkin) and R56 AI130130 (J.L.S.). S.J.E. was supported by grant 5U24AI116833-02 and the Bill and Melinda Gates Foundation OPP1155863. M.J.M. and S.J.E were supported by a grant from the Value of Vaccine Research Network. S.B.B. was partially supported as an Open Philanthropy Awardee of the Life Sciences Research Foundation. GM/CA-XSD is funded by the NIH National Institute of General Medical Sciences (AGM-12006) and the NIH National Cancer Institute (ACB-12002). The Eiger 16M detector at GM/CA-XSD was funded by the NIH (S10OD012289). This research used resources of the Advanced Photon Source, an Office of Science User Facility operated for the U.S. DOE Office of Science by the Argonne National Laboratory under contract no. DE-AC02-06CH11357.
© 2021 The Regents of the University of Michigan
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
