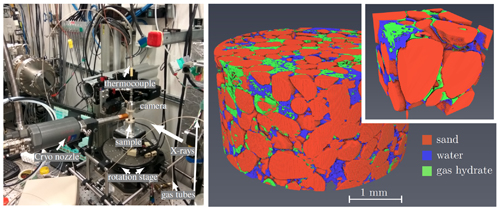
Methane hydrates are abundant in nature under conditions of high pressure and low temperature. They are important as a resource of clean-burning hydrocarbon fuel, but have a negative environmental impact if the methane dissociates and enters the atmosphere. Dynamic in situ three-dimensional (3-D) x-ray imaging, with short scanning times and high-resolution, improves researchers’ understanding of the chemical and physical processes of gas-hydrate formation and dissolution, which is crucial for developing safer and more efficient techniques for methane hydrate exploration and utilization. This work, published in the journal of Marine and Petroleum Geology, attempts to better understand the formation and dissociation of methane gas hydrates using synchrotron tomography at the U.S. Department of Energy’s Advanced Photon Source (APS). A technique new to the study of gas hydrates―four-dimensional x-ray imaging―revealed the time evolution of hydrate morphology, matrix composition, pore structure, and the hydrates’ effects on the physical properties of rocks. Using short scanning times and high resolution, the high-brightness x-rays from the APS revealed fast processes, i.e., water movement and gas hydrate formation and dissociation.
Methane hydrates―solid crystals of methane enclosed in a cage of water molecules―are most common in permafrost and deep-ocean sediments under conditions of high pressure (>35 bars) and low temperature (<10 oC). In the oceans, microorganisms convert organic materials into methane deep in a sediment pile. Deep sediments are too warm for hydrate stability, so the methane bubbles up into cooler sediments, where it is incorporated into methane hydrates.
Methane hydrates are plentiful along continental margins where organic material is abundant and temperature and pressure conditions are favorable. If conditions change, for instance, the ocean temperature rises, the hydrate structure dissociates and releases the methane (a potent greenhouse gas) into the atmosphere. Interest in methane hydrates is high because they are a clean-burning hydrocarbon fuel and are more abundant than conventional and shale resources combined. Methane that escapes into the atmosphere is a greenhouse gas and may contribute to the rise in global temperatures. However, researchers must learn more about chemical and physical processes during gas-hydrate formation and dissolution in order to develop efficient and safe techniques for utilizing them.
First, the researchers in this study filled the environmental cell with wet sand and introduced methane gas via high-pressure tubes at the top and bottom of the cell, under controlled temperature and gas pressure. Next, they used dynamic in situ three-dimensional imaging to study the rock structure during the formation/dissociation cycle using x-ray computed tomography at the X-ray Science Division 2-BM x-ray beamline of the APS, an Office of Science user facility at Argonne National Laboratory. To get the desired spatial and temporal resolution, the acquisition time for one data set ranged from one-half minute to several minutes.
High-resolution and variable scanning times of geologic sediments exposed complicated structures with small pores and mineral particles, gas, and multi-phase liquids. In situ imaging also revealed dynamic processes during gas-hydrate formation and dissociation. In addition to the previously known shell formation, gas hydrates form in gas pockets or inside water volumes. The researchers observed short, fast movements of water punctuated by periods of water immobility that may have been caused by cryogenic water suction during hydrate formation.
Dissociation in self-preservation mode (pressure drop at negative temperature) is different for each type of gas hydrate. Hydrates formed in water volumes are more stable than those formed in gas pockets, so each dissociates at a different speed. Interestingly, the stability of gas-hydrate accumulations and their production rates depends on their history prior to formation.
In the future, faster imaging will improve the researchers’ ability to understand the kinetics of gas hydrate formation and dissociation, which could optimize the methodology of methane hydrate production. Performing the experiments in a larger chamber will allow the researchers to measure the acoustic properties of the samples during methane hydrate formation. This will help them develop methods to interpret geophysical data for acoustic well logging and seismic exploration. ― Dana Desonie
See: Viktor V. Nikitin1*, Geser A. Dugarov2, Anton A. Duchkov2,3, Mikhail I. Fokin2, Arkady N. Drobchik2, Pavel D. Shevchenko4, Francesco De Carlo4, and Rajmund Mokso1, “Dynamic in-situ imaging of methane hydrate formation and self-preservation in porous media,” Mar. Petrol. Geol. 115, 104234 (2020). DOI: 10.1016/j.marpetgeo.2020.104234
Author affiliations: 1Lund University, 2Institute of Petroleum Geology and Geophysics SB RAS, 3Novosibirsk State University, 4Argonne National Laboratory
Correspondence: * viktor.nikitin@maxiv.lu.se, vnikitin@anl.gov
The work is supported by the Swedish Research Council grant (2017-00583). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being. ― Stephen Taylor
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
