The original Carnegie Institution for Science press release can be read here.
When a meteorite hurtles through the atmosphere and crashes to Earth, how does its violent impact alter the minerals found at the landing site? What can the short-lived chemical phases created by these extreme impacts teach scientists about the minerals existing at the high-temperature and pressure conditions found deep inside the planet? New work led by Sally June Tracy of the Carnegie Institution for Science used the U.S. Department of Energy’s Advanced Photon Source (APS) to examine the crystal structure of the silica mineral quartz under shock compression and is challenging longstanding assumptions about how this ubiquitous material behaves under such intense conditions. The results were published in Science Advances.
"Quartz is one of the most abundant minerals in Earth’s crust, found in a multitude of different rock types,” Tracy explained. “In the lab, we can mimic a meteorite impact and see what happens.”
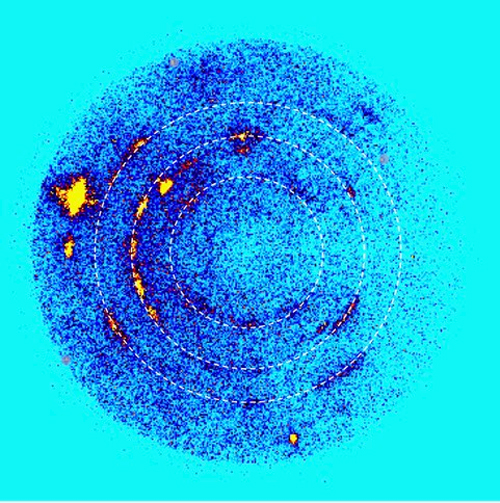
Tracy and her colleagues—Washington State University’s (WSU) Stefan Turneaure and Princeton University’s Thomas Duffy—used specialized impact facilities to accelerate projectiles into quartz samples at extremely high speeds—several times faster than a bullet fired from a rifle. Special x-ray instruments were used to discern the crystal structure of the material that forms less than one-millionth of a second after impact (Fig.1). Experiments were carried out at the Dynamic Compression Sector (DCS), which is operated by WSU and located at Sector 35 of the APS, an Office of Science user facility at Argonne National Laboratory.
Quartz is made up of one silicon atom and two oxygen atoms arranged in a tetrahedral lattice structure. Because these elements are also common in the silicate-rich mantle of the Earth, discovering the changes quartz undergoes at high-pressure and -temperature conditions, like those found in the Earth’s interior, could also reveal details about the planet’s geologic history.
When a material is subjected to extreme pressures and temperatures, its internal atomic structure can be re-shaped, causing its properties to shift. For example, both graphite and diamond are made from carbon. But graphite, which forms at low pressure, is soft and opaque, and diamond, which forms at high pressure, is super-hard and transparent. The different arrangements of carbon atoms determine their structures and their properties, and that in turn affects how we engage with and use them.
Despite decades of research, there has been a long-standing debate in the scientific community about what form silica would take during an impact event, or under dynamic compression conditions such as those deployed by Tracy and her collaborators. Under shock loading, silica is often assumed to transform to a dense crystalline form known as stishovite—a structure believed to exist in the deep Earth. Others have argued that because of the fast timescale of the shock the material will instead adopt a dense, glassy structure.
Tracy and her colleagues used the uniquely integrated x-ray and shock wave capabilities at the DCS to probe in real time the atomic structure of quartz subjected to a dynamic shock of greater than 300,000 times normal atmospheric pressure. The results demonstrate that, counter to expectations, the shock-compressed quartz undergoes a transition to a novel disordered crystalline phase, whose structure is intermediate between fully crystalline stishovite and a disordered glass. However, the new structure cannot last once the burst of intense pressure has subsided.
“Dynamic compression experiments allowed us to put this longstanding debate to bed,” Tracy concluded. “What’s more, impact events are an important part of understanding planetary formation and evolution and continued investigations can reveal new information about these processes.”
See: Sally June Tracy1,2*, Stefan J. Turneaure3, and Thomas S. Duffy1, “Structural response of α-quartz under plate-impact shock compression,” Sci. Adv. 6, eabb3913 (26 August 2020). DOI: 10.1126/sciadv.abb3913
Author affiliations: 1Princeton University, 2Carnegie Institution for Science, 3Washington State University
Correspondence: *sjtracy@carnegiescience.edu
We thank J. Klug, Y. Li, D. Rickerson, A. Schuman, N. Sinclair, P. Rigg, and B. Williams of the Dynamic Compression Sector for assistance with the impact experiments. This research was supported by the Defense Threat Reduction Agency (HDTRA1-15-1-0048) and the National Science Foundation (EAR-1644614). Washington State University provided experimental support through the U.S. Department of Energy (DOE)/National Nuclear Security Agency (NNSA) Award nos. DE-NA0002007 and DE-NA0003957. This work is based on experiments performed at the Dynamic Compression Sector, operated by WSU under DOE/NNSA Award no. DE-NA0002442. This research used the resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by the Argonne National Laboratory under contract no. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
