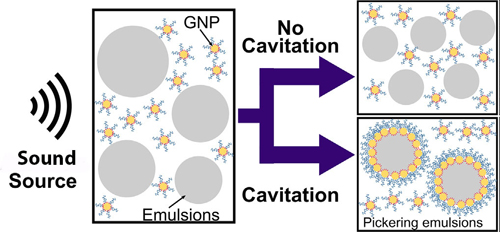
Homogenized milk, skin cream, and mayonnaise are just a few examples of Pickering emulsions, mixtures of oil and water stabilized with solid particles. Pickering emulsions are just as common in cosmetics, agrochemicals, and drug delivery systems as they are in food preparations, and for the same reasons: instead of using a surfactant (like a soap) to keep oil and water mixed, they use a particle such as a protein or metal oxide that can have additional functionality beyond simply keeping the emulsion together. Despite how common Pickering emulsions are, the mechanics of how they form are not well understood. Multiple repulsive forces involving electrical charge or particles shape work to prevent emulsification. Mechanical forces such as shaking or ultrasound waves have to be applied to overcome the repulsions. Researchers used the U.S. Department of Energy’s Advanced Photon Source (APS) to understand how sound waves overcome the energy barriers to Pickering emulsification. Their findings should lead to more efficient manufacturing of drug, food, and chemical emulsions, as well as medical applications, in the future.
Pickering emulsions using perfluorinated oils have recently gained a following in the medical community as potential imaging agents. Using a combination of ultrasound and laser pulses, these emulsions can simultaneously provide contrast and break up blood clots, making them potentially useful for doctors treating victims of stroke and embolism. Researchers from the University of Washington teamed up with colleagues at Argonne National Laboratory to investigate exactly how sonication — that is, exposing the ingredients to powerful sound waves — creates Pickering emulsions of these valuable medical compounds. They found suggestive evidence that cavitation, meaning the creation of bubbles, may be the key.
The researchers chose gold nanoparticles as the solid particles that would stabilize the Pickering emulsion. Onto the gold nanoparticles they added polyethylene glycol molecules with a hydrophilic (“water-loving”) end, and then added hydrophobic (“oil-loving”) molecules to the other side of the nanoparticles. Then they gently mixed the nanoparticles with water and perfluorinated oils, and waited.
But no matter how long they waited, the mixture did not emulsify. The water and perfluorinated oil stayed in separate layers. It did not spontaneously form a Pickering emulsion, showing that the mere presence of stabilizing particles was insufficient to make the emulsion happen. The researchers also tried mixing with a magnetic stirrer, which caused some shear in the liquid, but this did not lead to emulsification either.
But when they tried sonicating the mixture, everything came together. Rapidly moving sound waves of high enough acoustic pressure create cavitation (bubbles) in the low-pressure areas, as the quick drop in pressure causes some of the liquid to evaporate. When these bubbles subsequently collapse, they create tiny little shock waves.
When the researchers sent sound waves above 7.2 megapascals (MPa) through the mixture, it quickly formed a Pickering emulsion of droplets of oil in water and the droplets were stabilized by the gold nanoparticles. However, the mixture did not emulsify when sonicated at sound pressures below 6.4 MPa, which is too low to create cavitation (Fig. 1.) This indicated that bubbles are essential to the Pickering emulsion process.
To better understand exactly what was changing structurally within the mixture as it became a Pickering emulsion, the team used the X-ray Science Division 9-ID-C beamline at the APS, an Office of Science user facility at Argonne. They employed the ultra small-angle x-ray scattering (USAXS) technique, which uniquely provides the ability to quantitatively measure structural changes while applying sound waves. The APS had the capacity to design a custom acoustic environment in which the sound pressure, sound pulse frequency, and pulse duration could all be precisely controlled.
The USAXS data showed that sonication at sound pressures too low to create cavitation was actually bad for the Pickering emulsion, destabilizing the oil and causing it to evaporate without reforming droplets. The emulsion lost oil that way.
From analyzing the USAXS data, the researchers now have two specific hypotheses as to how Pickering emulsification occurs. The first assumes that cavitation happens in the water, and then momentum from the shock waves of collapsing bubbles breaks up the oil and encourages the gold nanoparticles to adsorb onto the oil droplets. The second hypothesis assumes, instead, that cavitation happens in the oil. Oil bubbles expand very quickly and then collapse. The high velocity and displacements that occur during the rapid expansion and collapse could entrap the gold nanoparticles and induce them to adsorb onto the surface of the oil droplet.
The researchers hope to use the time-resolved small-angle x-ray scattering technique to tease out these different scenarios in the next stage of their research. But based on the results they already have, they suggest that manufacturers of medical Pickering emulsions should seek to use a lower-frequency sonication method to minimize oil evaporation and loss, or consider using a pre-emulsification treatment to create smaller oil droplets to start with before beginning the Pickering emulsification process. – Kim Krieger
See: Yi-Ting Lee1, David S. Li1, Jan Ilavsky2, Ivan Kuzmenko2, Geng-Shi Jeng1, Matthew O’Donnell1, and Lilo D. Pozzo1, “Ultrasound-based formation of nano-Pickering emulsions investigated via in-situ SAXS,” J. Colloid Interf. Sci. 536 281, (2019). DOI: 10.1016/j.jcis.2018.10.047
Author affiliations: 1University of Washington, 2Argonne National Laboratory
Correspondence: *dpozzo@uw.edu
Acknowledgment is made to the Donors of the American Chemical Society Petroleum Research Fund for support of this research. The research performed was also supported by the National Institutes of Health under grant R01HL125339. We also acknowledge Yuyin Xi for his help collecting scattering data at Argonne National Laboratory. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy's APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
