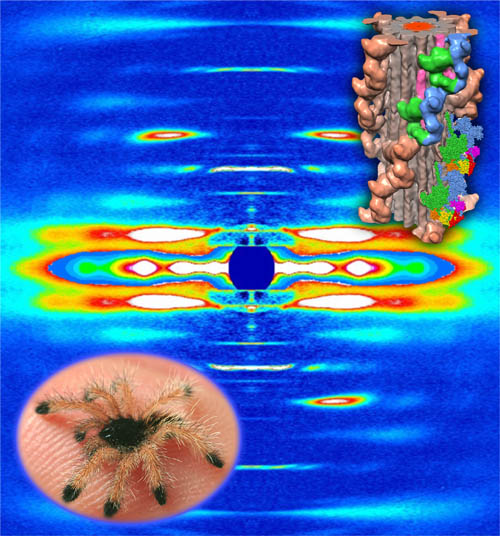
The human heart is a remarkable feat of evolutionary engineering. Beating about 100,000 times per day and pumping nearly 2,000 gallons of blood through an interconnected series of veins, arteries, and capillaries that spans a distance greater than 60,000 miles, the heart is the most important muscle in the human body. Yet, heart disease remains the number one cause of death in the world, demonstrating the need for more research in heart physiology. Now a research team has found an unlikely source of inspiration for understanding how the human heart works and how we might design better drugs for conditions like hypertrophic cardiomyopathy: tarantulas. The source of nightmares for arachnophobes and the household pets for arachnophiles are inspiring researchers to take new approaches to understanding diseases that alter how heart muscle cells contract and relax. But, before getting to the human heart, there is more to learn about the physiology of tarantula muscles. The researchers set out to understand how contractions in tarantula muscle cells are activated and why are muscle twitches that follow a sustained muscle contraction (post-tetanic) more forceful than those that don’t (pre-tetanic). Some of their key findings were based on x-ray diffraction experiments conducted at the U.S. Department of Energy’s Advanced Photon Source (APS). Their results provide evidence that phosphorylation, the chemical addition of a phosphoryl group (PO3-) to an organic molecule, plays a key role in muscle activation and post-tetanic potentiation (PTP) in tarantula muscles.
Striated muscles are composed of thick and thin filaments that contain myosin and actin proteins, respectively. When activated, these two fibrous proteins interact to cause their relative sliding, resulting in the production of force and muscle shortening. In the fully relaxed phase, the researchers from the University of Massachusetts Medical School, the Illinois Institute of Technology, the Instituto Venezolano de Investigaciones Científicas (Venezuela), and Moscow State University (Russia) used x-ray diffraction (XRD) obtained at the Biophysics Collaborative Access Team x-ray beamline 18-ID at the APS to show that neighboring free and blocked myosin heads on the thick filament are arranged in an interacting-heads motif (IHM) within live tarantula muscles, supporting previous results obtained by cryogenic electron microscopy as seen in Fig. 1. In the case of fully relaxed muscles, there is little evidence of myosin phosphorylation before the tetanic contraction. (The APS is an Office of Science user facility at Argonne National Laboratory.)
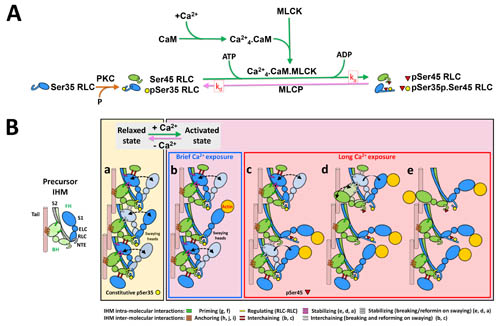
To understand the activation of myosin proteins, the researchers monitored time-resolved XRD experiments on excised tarantula leg muscles. Results indicate that phosphorylation of the myosin regulatory light chain (RLC) allows the myosin heads to move away from the thick filament and towards actin proteins on the thin filament to initiate binding. Blocked myosin heads are mono-phosphorylated and slowly move away from the thick filament whereas the free myosin heads are bi-phosphorylated and move completely away. This mechanism is illustrated in Fig. 2. By the end of the tetanic event, urea-glycerol gel electrophoresis and XRD results show a substantial increase in mono and bi-phosphorylation levels of the myosin proteins with almost no evidence of non-phosphorylated myosin — indicating that phosphorylation is the first step in activating the myosin proteins.
The full relaxation time for tarantula leg muscles can take longer than 6 minutes depending on the level of phosphorylation. During this process, the mono-phosphorylated blocked myosin heads become dephosphorylated and return to the thick filament and the fully released bi-phosphorylated myosin heads become mono-phosphorylated and slowly dock back to the thick filament. Prior to such full relaxation, the myosin heads remain partially phosphorylated and away from the thick filament and readily available for interaction with actin, making it easier to produce force during a post-tetanic twitch. This appears to be the basis of post-tetanic twitch potentiation. These results are supported by the decline in PTP over time, as the RLC phosphorylation gradually returns to the baseline. Collectively, the researchers conclude that the activation and PTP behavior of tarantula muscles are consistent with an IHM Cooperative Phosphorylation Activation mechanism (Fig. 2).
Interestingly, the mechanism for myosin activation in tarantulas is different than what has been observed in other species. There are examples in the literature that show direct binding of calcium ions, mechanosensing, or delayed stretch activation as the main activation step for releasing myosin so that it can bind with actin on the thin filament to start a muscle contraction.
Evolution has a way of slowly adapting our physiologies to best match our environmental conditions. A tarantula, which has a very sedentary lifestyle with small bursts of movement for capturing prey, may exhibit different muscle physiology than a frog.
Ultimately, understanding the ways that muscles contract and relax in many different species will provide a deeper understanding of how to protect the most important muscle in our bodies. ― Stephen Taylor
See: Raúl Padrón1*, Weikang Ma2, Sebastian Duno-Miranda3, Natalia Koubassova4, Kyoung Hwan Lee1, Antonio Pinto3, Lorenzo Alamo3, Pura Bolaños3, Andrey Tsaturyan4, Thomas Irving2, and Roger Craig1, “The myosin interacting-heads motif present in live tarantula muscle explains tetanic and posttetanic phosphorylation mechanisms,” Proc. Natl. Acad. Sci. 117(22), 11865 (June 2, 2020). DOI: 10.1073/pnas.1921312117
Author affiliations: 1University of Massachusetts Medical School, 2Illinois Institute of Technology, 3Instituto Venezolano de Investigaciones Científicas, 4Moscow State University
Correspondence: *raul.padron@umassmed.edu
Research was supported by National Institutes of Health Grants National Institute of General Medical Sciences GM103622 (to T.I.), National Heart, Lung, and Blood Institute HL139883 (to Richard Moss and R.C.), National Institute of Arthritis and Musculoskeletal and Skin Diseases AR072036 (to R.C.), AR067279 (to R.C. and D. Warshaw), Office of Research Infrastructure Programs 1S10OD018090-01 (to T.I.), and Russia State Program AAAA-A19-119012990119-3 (to N.K. and A.T.). This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
