Exposure to hydrogen gas (H2) causes the embrittlement of steel, a phenomenon first observed nearly 150 years ago. Embrittlement means that the steel's ductility is reduced. Although this process has been extensively examined using a variety of analytical techniques, such as optical imaging and electron microscopy, the mechanisms, or combination of mechanisms, that are chiefly responsible for hydrogen-induced embrittlement remain unclear. One of the challenges encountered when investigating hydrogen embrittlement is that the sample being examined is usually removed from the hydrogen environment prior to analysis. This precludes real-time imaging of the hydrogen/steel dynamic. To address this limitation, researchers using the U.S. Department of Energy’s Advanced Photon Source (APS) carried out the first application of high-energy x-ray diffraction (HEXD) to a steel sample during H2 exposure. Analysis indicates that the hydrogen-induced embrittlement arose from a combination of two distinct mechanisms. Understanding the primary mechanisms responsible for steel embrittlement caused by H2 exposure should help materials scientists develop strategies to ameliorate this problem, which has important ramifications for hydrogen storage and transport, as well as being a prerequisite for realizing a future hydrogen economy.
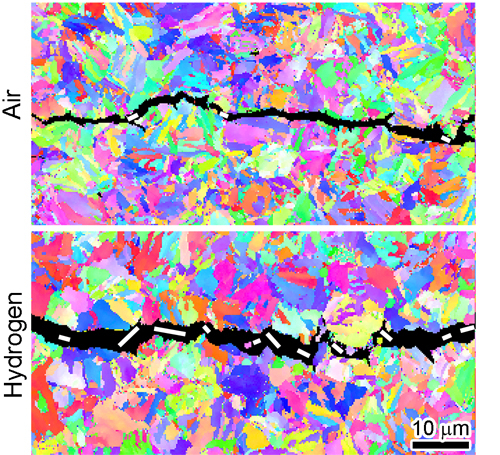
External forces acting on a steel object are translated into microscopic deformations and stresses within its polycrystalline structure. If the magnitude of these stresses is sufficiently large, microscopic fatigue cracks will form and propagate over time. Crucially, under the same external loading conditions, any fatigue cracks exposed to hydrogen will grow 10 to 100 times faster than those exposed to air. Microscopic fatigue cracks in steel are shown in Fig. 1. The crack in the upper panel formed in air, while the lower crack formed in H2.
Numerous mechanisms have been proposed to explain the significantly higher fatigue crack growth observed in steels exposed to hydrogen. The two mechanisms tested in this study were hydrogen enhanced decohesion (HEDE) and hydrogen enhanced localized plasticity (HELP). The HEDE model explains increased crack growth as resulting from hydrogen promoting decohesion within the steel. Broadly speaking, decohesion refers to the loosening of atomic or molecular bonds. Sufficient decohesion allows a crack to propagate and can occur in two ways: Decohesion within individual grains is referred to as transgranular decohesion (labeled T-HEDE), while decohesion between grains is called intergranular decohesion (IG-HEDE).
The HELP model invokes a different mechanism to explain hydrogen-enhanced crack growth. According to HELP, the presence of hydrogen facilitates the movement of dislocations within the grains (individual crystals) of the steel. A dislocation is a mobile line defect in a grain's crystalline structure whose movement causes plastic deformation. The presence of dislocations within individual grains can actually be helpful by alleviating stress buildup, thereby limiting fatigue crack nucleation and growth. However, the presence of hydrogen can cause redistribution of the dislocations within steel grains, causing a crucial buildup in strain that enhances crack propagation.
Over the years, materials scientists have cited different experimental results to promote either the HEDE or HELP models. However, recent studies favor a more balanced approach in which the HEDE and HELP mechanisms are seen as complimentary, with both playing a role in explaining hydrogen's deleterious effect on crack formation and growth.
To unravel which mechanisms play significant roles in hydrogen embrittlement, samples of the low-alloy steel AISI 4130 were subjected to several experimental tests, including high energy x-ray diffraction (HEXD) experiments at the X-ray Science Division 1-ID-E x-ray beamline at the APS, an Office of Science user facility at Argonne National Laboratory. In the HEXD protocol, 4130 steel samples were placed in either air or hydrogen environments. The samples were then cyclically stretched and relaxed to induce microscopic fatigue cracks.
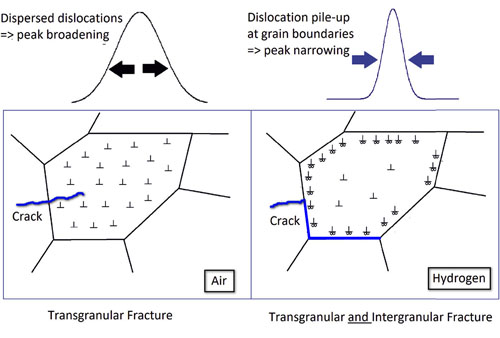
Figure 2 illustrates some of the HEXD results. Broadening of the diffraction peaks occurred for the sample in air (left side of Fig. 2), indicating that dislocations were spread more-or-less evenly across the entire grain. In contrast, the narrower diffraction peaks recorded for the sample in hydrogen (right side of Fig. 2) indicate an accumulation of dislocations at the grain boundary. The accumulation of dislocations supports the HELP model of H2-induced embrittlement. However, the fact that the fracture in the hydrogen environment (right side of Fig. 2) also appeared between the grain boundary, i.e., was intergranular, lends support to the intergranular decohesion, or IG-HEDE, model.
The experimental results indicate that both the HEDE and HELP mechanisms play important roles in the hydrogen embrittlement of steel. Moreover, the HEXD data should also prove useful in calibrating models of fatigue crack formation for use in future studies. ― Philip Koth
See: Matthew Connolly1*, May Martin1, Peter Bradley1, Damian Lauria1, Andrew Slifka1, Robert Amaro2, Christopher Looney3, and Jun-Sang Park4, “In situ high energy X-ray diffraction measurement of strain and dislocation density ahead of crack tips grown in hydrogen,” Acta Mater. 180, 272 (2019). DOI: 10.1016/j.actamat.2019.09.020
Author affiliations: 1National Institute of Standards and Technology, 2Southern Research, 3Colorado School of Mines, 4Argonne National Laboratory
Correspondence: * matthew.connolly@nist.gov
This work was supported by the U.S. Department of Transportation under grant DTPH5615X00015 and the U.S. Department of Energy (DOE) contract DE-NA-0003525. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The U.S. Department of Energy’s Advanced Photon Source (APS) at Argonne National Laboratory is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
