Supramolecular structures are complex arrays of molecules held together by comparatively weak intermolecular bonds. The study of these nano-assemblies can help us learn more about the fundamentals of chemistry and physics, and they also have significant applications in medicine and energy science. In recent experimentation at the U.S. Department of Energy’s (DOE’s) Advanced Photon Source (APS), a research team made an important discovery directly pertinent to nano and clean-energy science. By chemically modifying a photosensitive organic dye (perylene monoimide), the authors of this study created a supramolecular system that is water soluble and can be controlled by altering the acidity (i.e., pH) of this system’s environment. They additionally showed that these assemblies can be used to drive light-induced reactions, suggesting exciting possibilities for the future of renewable energy.
Perylene monoimides are a type of organic dye that is capable of absorbing visible light. An “imide” is an organic functional group possessing the structure (—CONHCO—) built around a central nitrogen atom. In contrast, perylene is a unique structure compromised of multiple different carbon rings. Its chemical formula is C20H12 and its official chemical type is a polycyclic aromatic hydrocarbon. Thus, a perylene monoimide dye is a dye that contains both a multi-ringed structure (i.e., the perylene hydrocarbon) and a unique chemical group that contains nitrogen and oxygen (i.e., the imide). Imides are well known for their presence in high-strength commercial polymers while perylene has been utilized in research as a probe to measure the presence of fat molecules.
In this study, the researchers from Northwestern University investigated a unique, water-soluble perylene monoimide system that was carboxylated, meaning that a carboxyl group (R–COOH) was added to the dye. Additionally, the authors functionalized the core of the perylene molecule with a hydroxyl group (a molecule with oxygen and hydrogen bonded together). This made the entire supramolecular assembly ionizable, meaning that hydrogen atom of the hydroxyl group can be reversibly exchanged off and onto the perylene core in response to the solutions acidity level. The pH level of a given environment can range from 0 (most acidic) to 14 (most basic). The authors showed that these assemblies could be used as photosensitizers: they can harness and transfer energy from light to produce chemical changes. Specifically, they demonstrated that the photocatalytic production of hydrogen (combination of two protons to create a molecule of hydrogen gas) was possible at a pH of 4. This system was also capable of converting carbon dioxide to higher value products at a more alkaline pH of 9-10.
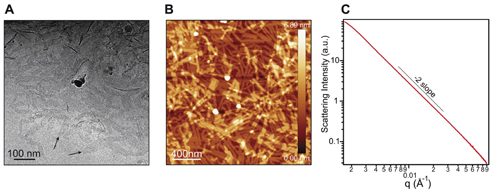
A significant portion of this work required the Advanced Photon Source at Argonne National Laboratories. More specifically, solution x-ray experiments were conducted at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) 5-ID-B,C,D x-ray beamline at the APS; and at the X-ray Science Division 8-ID-E beamline, also at the APS. (The APS is an Office of Science user facility at Argonne National Laboratory.) X-ray scattering was performed to characterize the nanostructure morphology of the customized perylene monoimide supramolecular system. The researchers used solution phase wide-angle x-ray scattering to probe differences in the internal structure of the nano-assemblies as a function of pH. Cryogenic transmission electron microscopy (cryo-TEM) and atomic force microscopy (AFM) helped reveal the morphology of the supramolecular nanoscale ribbons. Small angle x-ray scattering (SAXS) was then used to confirm the ribbon morphology observed by microscopy. The data for these studies are shown in Fig. 1.
While there are several different theoretical applications for this work, the most cogent application is relevant to the fields of energy science and renewable energy. The ability to utilize light to drive chemical reactions (photosynthesis) is the basis of life on our plant, and the ambitious goal of splitting water into hydrogen to generate energy is longstanding. This edifying work has helped to showcase another potential source of renewable solar energy, which puts us collectively one-step closer to a greener, safer planet. ― Alicia Surrao
See: Adam Dannenhoffer, Hiroaki Sai, Dongxu Huang, Benjamin Nagasing, Boris Harutyunyan, Daniel J. Fairfield, Taner Aytun, Stacey M. Chin, Michael J. Bedzyk, Monica Olvera de la Cruz, and Samuel I. Stupp*, “Impact of charge switching stimuli on supramolecular perylene monoimide assemblies,” Chem. Sci. 10, 5779 (2019). DOI: 10.1039/c8sc05595e
Author affiliation: Northwestern University
Correspondence: *s-stupp@northwestern.edu
This work was supported as part of the Argonne-Northwestern Solar Energy Research (ANSER) Center, an Energy Frontier Research Center funded by the U.S. Department of Energy (DOE) Office of Science-Basic Energy Sciences under Award # DE-SC0001059. Determination of Electronic Energy levels and DFT calculations were supported by the U.S. DOE Office of Science-Basic Energy Sciences, under award no. DE-FG02-00ER45810. Molecular dynamic simulations were possible thanks to the generous support from the U.S. DOE under award no. DE-FG02-08ER46539. DND-CAT is supported by Northwestern University, E.I. DuPont de Nemours & Co., and The Dow Chemical Company. The authors thank Dr J. Strzalka for help with GIWAXS setup and measurements at Argonne National Lab. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
