Parkinson's disease (PD) is a neurodegenerative disorder that affects about 7 million people worldwide, including 1 million in the U.S. Though the causes of PD remain unknown, the leucine-rich repeat kinase 2 (LRRK2) protein is a known contributor to both sporadic and inherited disease. Importantly, the C-terminal of LRRK2, the WD40 domain, is required for LRRK2 neurotoxicity. To better understand the biological functions of LRRK2 and the impact that PD-associated mutations have on protein function, researchers used anomalous diffraction phasing at the U.S. Department of Energy’s Advanced Photon Source (APS) to solve the structure of the WD40 domain of human LRRK2 to a resolution of 2.6 angstroms. This structure allowed researchers to map known disease-associated mutations and to use complimentary biochemical analysis to explore the functional effects of pathogenic mutations on LRRK2 dimerization. Together, these findings provide support for development of LRRK2-targeting therapies for PD.
There are a number of genetic and environmental factors associated with development of Parkinson’s disease. For example, the incidence of PD increases with age, and there are a number of mutations within a range of genes that have been associated with PD. Though mutations in some of these genes are present in a small subset of PD patients, like those responsible for familial PD, others, like the protein LRRK2, are major contributors to hereditary and sporadic PD.
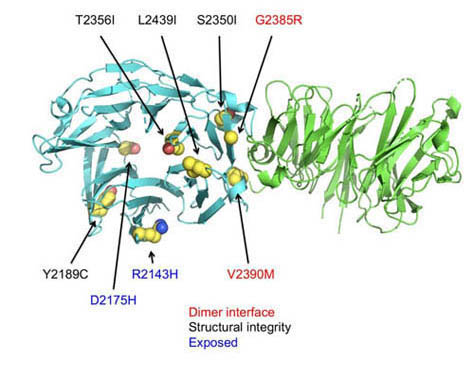
Though the exact function of LRRK2 is unclear, as is the exact nature of its contribution to PD, it is known that the C-terminal WD40 structural domain is required for LRRK2-indiced neurotoxicity. In the current work, the researchers from Harvard Medical School, Boston Children’s Hospital, the University of Dundee (UK), and the University of California, San Diego, crystalized WD40 and used x-ray diffraction data obtained at the Northeastern Collaborative Access Team 24-ID-C beamline at the APS to build a model of the protein to a resolution of 2.6 Å. (The APS is an Office of Science user facility at Argonne National Laboratory.) With the structure of WD40 in hand, the researchers were able to map mutations known to contribute to PD onto the three-dimensional structure (Fig. 1). Using complementary biochemical analysis, the team confirmed that LRRK2 exists in solution as a concentration-dependent dimer, resolving ambiguity in the field surrounding the functional state of the protein. Importantly, several mutations at the dimerization interface were shown to increase LRRK2 activity, lending support to the hypothesis that LRRK2 pathogenicity in PD is due to hyperactivation of the protein.
That PD mutations contribute to hyperactivation is an important finding, as this supports LRRK2 as a drug target for novel anti-PD therapies. Future research will be aimed at a deeper understanding of the complexities of LRRK2 biology, including the factors which influence the imperfect correlation between LRRK2 dimerization and activity, and the effect of pathogenic mutations on the activity of the full-length protein. — Emma Nichols
See: Pengfei Zhang1,2, Ying Fan3, Heng Ru1,2, Li Wang1,2, Venkat Giri Magupalli1,2, Susan S. Taylor4, Dario R. Alessi3, and Hao Wu1,2*, “Crystal structure of the WD40 domain dimer of LRRK2,” Proc. Natl. Acad. Sci. U.S.A. 116(5), 1579 (2019). DOI: 10.1073/pnas.1817889116
Author affiliations: 1Harvard Medical School, 2Boston Children’s Hospital, 3University of Dundee, 4University of California, San Diego
Correspondence: * wu@crystal.harvard.edu
This work was supported by The Michael J. Fox Foundation for Parkinson’s Research [Grants 11211 (to H.W.) and 6986 (to D.R.A.)] and by the Medical Research Council [Grant MC_UU_12016/2 (to D.R.A.)]. The NE-CAT beamlines are funded by the National Institute of General Medical Sciences from National Institutes of Health (NIH) Grant P30 GM124165. The Pilatus 6M detector on the 24-ID-C beamline is funded by a NIH-Office of Research Infrastructure Programs High-End Instrumentation Grant S10 RR029205. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357.
The APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
