Materials science tends to divide solids into two basic types: conductors and insulators. Conductors transport energy efficiently in the form of heat or electricity. Insulators do not. And the higher the temperature, the worse an insulator is at transporting energy. But not all materials fall neatly into one of these two categories, and those exceptions can sometimes be useful. For example, materials that conduct electricity but insulate heat can be used for converting heat into electricity and are called thermoelectrics. A team of researchers used the APS to study one such promising class of materials, lead chalcogenides, and confirmed what their simulations predicted: As the temperature rose, certain frequencies of vibration (heat) moved through the material more efficiently, while others shut down. Their work could help materials scientists better understand why some substances have unusual properties, and eventually identify or design materials that can gather waste heat and put it to work elsewhere. Ultimately, such materials could convert waste heat into electricity, or replace mechanical cooling systems that rely on fossil fuels with more environmentally friendly solid-state devices.
Lead selenide (PbSe) transports heat badly because its crystalline structure doesn’t vibrate in a typical harmonic way. Instead, the crystals vibrate in complicated modes that interact in unexpected ways and sometimes localize, i.e., they stop moving heat through the crystal.
Researchers from Caltech simulated the molecular dynamics of PbSe, explicitly accounting for the anharmonic behavior of the material, to see if they could get some insight into its heat transport. In a typical insulator, the modes of vibration interact more and more as the material gets warmer and warmer. These interactions interfere with heat transport. But surprisingly, the simulation did not find this to be entirely true for PbSe. Instead, the simulation showed that at high temperature one of the modes of vibration split off from the others, not propagating at all, while another traveled more freely.
The Caltech group teamed up with a researcher from Oak Ridge National Laboratory (ORNL) who heated a sample of PbSe while passing a beam of neutrons through it at the NIST Center for Neutron Research. At room temperature, the researchers saw several different modes of vibration, indicated by how the energy and direction of the neutrons changed after passing through the sample. But as the PbSe warmed, they not only observed the transverse optical (TO) phonon mode flatten out and stop propagating just as the simulation predicted, they also observed sharpening of the longutudinal acoustic (LA) phonon mode. On the other hand, instrumental resolution prevented qualitative analysis of the predicted splitting of TO mode from intrinsic localized mode (ILM) and made determination of the energy linewidth of LA mode challenging. High-energy-resolution measurements were later performed on the same crystal using the HYSPEC time-of- flight cold neutron spectrometer at the Spallation Neutron Source at ORNL in order to look for any fine-energy structure. These measurements revealed an additional sharp but weak dispersionless feature matching the in-band ILM feature and shows a flatter, more fragmented TO phonon, as predicted in their simulation, meaning a transition into the anharmonic dynamics upon heating.
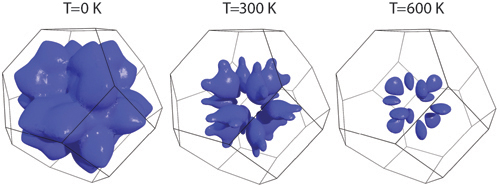
The team wanted to take a closer look at those modes that disappeared, so to view the vibrational modes of interest as the PbSe was slowly warmed, they and a colleague from Argonne carried out inelastic x-ray scattering studies at the HERIX spectrometer on the XSD 30-ID-C beamline of the APS, the only synchrotron in North America that will work for this kind of experiment because it has the highest flux, the brightest x-rays, very little noise from randomly scattered rays, and better control over the energy of the x-rays than any other facility. What the researchers saw at the APS confirmed their simulation results and the neutron beam analysis: The velocity of certain vibrational modes shrank, gradually slowing to a stop as the temperature rose (Fig. 1). At room temperature (about 294 K) certain vibration modes interacted with other nearby modes fairly normally. But as the temperature rose to 770 K, these modes sharpened, interacting with other modes less and persisting for long periods of time. The reason this happens is that when one mode localized its movement in energy-momentum space, this reduced the number of possible scattering paths, allowing the other mode to pass more freely through the crystal. It is surprising because normally with increasing temperature, the thermal population of more vibrations increases the scattering, and it is usually assumed that the available scattering channels do not change.
The measurements at the APS confirmed what the neutron beam experiment had shown: The flattening out wasn’t just of certain isolated modes, but of whole swaths of vibrations in the material. Understanding how vibrations can be induced to stop propagating in a pristine crystal opens new avenues to control thermal transport without disrupting the crystal regularity needed for favorable electric properties in thermoelectric applications. The trick is dealing with the compensating effects that come with localization from changes in the phase space for scattering other vibrations.
These results tell us that materials can be more complex than researchers had thought. It also gives theorists an idea of which characteristics unusual thermoelectrics like PbSe should have, allowing scientists to seek out materials with even better properties, or design them from scratch. — Kim Krieger
See: M.E. Manley1*, O. Hellman2, N. Shulumba2, A.F. May1, P.J. Stonaha1, J.W. Lynn3, V.O. Garlea1, A. Alatas4, R.P. Hermann1, J.D. Budai1, H. Wang1, B.C. Sales1, and A.J. Minnich2**, “Intrinsic anharmonic localization in thermoelectric PbSe,” Nat. Commun. 10, 1928 (2019). DOI: 10.1038/s41467-019-09921-4
Author affiliations: 1Oak Ridge National Laboratory, 2California Institute of Technology, 3National Institute of Standards and Technology, 4Argonne National Laboratory
Correspondence: * manleyme@ornl.gov,
** aminnich@caltech.edu
This work was supported by the U.S. Department of Energy (DOE) Office of Science-Basic Energy Sciences, Materials Sciences and Engineering Division under Contract Number DE-AC05-00OR22725. A portion of this research performed at the Oak Ridge National Laboratory’s Spallation Neutron Source was sponsored by the U.S. DOE Office of Science-Basic Energy Sciences. The authors acknowledge the support of the National Institute of Standards and Technology, U.S. Department of Commerce, in providing the neutron research facilities used in this work. H. Wang’s effort was sponsored by the DOE Energy Efficiency and Renewable Energy, Office of Vehicle Technologies Materials program. N.S. and A.J.M. acknowledge the support of the DARPA MATRIX program under Grant No. HR0011-15-2-0039. This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation Grant No. ACI-1053575. This research used resources of the Advanced Photon Source, a U.S. DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
