 Inexpensive, abundant, and useful—pure aluminum metal is soft and ductile and perfect for end uses such as soda cans and aluminum foil. But add just a little bit of copper, magnesium, or zinc, and aluminum transforms into a super-strong yet lightweight material, stiff and resilient enough to be used in aircraft and automobile frames. Although we discovered how to alloy aluminum with other metals 60 years ago, we still don’t entirely understand how these small additions cause such dramatic changes in aluminum properties. Now, researchers have used the unique capabilities of the APS to detail for the first time how these metals change the nanoscale structure of aluminum, and to explore how we might control the metallurgy to design even better aluminum alloys with even more desirable properties.
Inexpensive, abundant, and useful—pure aluminum metal is soft and ductile and perfect for end uses such as soda cans and aluminum foil. But add just a little bit of copper, magnesium, or zinc, and aluminum transforms into a super-strong yet lightweight material, stiff and resilient enough to be used in aircraft and automobile frames. Although we discovered how to alloy aluminum with other metals 60 years ago, we still don’t entirely understand how these small additions cause such dramatic changes in aluminum properties. Now, researchers have used the unique capabilities of the APS to detail for the first time how these metals change the nanoscale structure of aluminum, and to explore how we might control the metallurgy to design even better aluminum alloys with even more desirable properties.
Metallurgists make aluminum alloys by dissolving a small amount of one or more metals—say 1% of copper by weight—into a larger body of aluminum. Once the addition is completely dissolved, the mixture is quenched, that is, cooled quickly so that it stays evenly mixed. Then the mix is slowly reheated just enough that tiny particles of Al2Cu precipitate out.
These particles are shaped like tiny needles or plates. Just a few tens of nanometers long, they give aluminum alloys their strength and stiffness. Material scientists knew the particles did this, but until now they didn’t know exactly how.
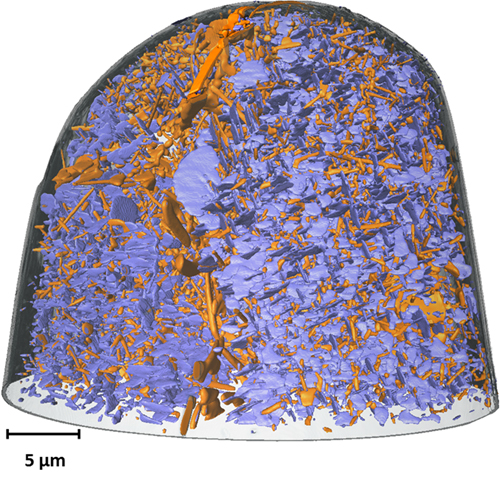
A team of researchers from Arizona State University used the APS to find out. They took a sample of aluminum copper alloy and heated it slowly to allow the copper to precipitate into nanoparticles of Al2Cu. The researchers, with colleagues from XSD, then milled pillars, using a focused ion beam, down to about 50 µm at the tip, and imaged these at the XSD x-ray beamline 32-ID-C at the APS. They used the transmission x-ray microscope at that beamline to perform absorption full-field hard x-ray nano-computed tomography. Similar to a medical computed tomography scan, the transmission x-ray microscope was used to take a series of two-dimensional images of the interior nanostructure of the wire, and then, using tomoPy, an open-source, Python-based toolbox developed at the APS, those images were reconstructed into a three-dimensional picture (Fig. 1) .
Once they had imaged the starting structure of the alloy, the team carefully applied pressure to the wire with a diamond tip until the wire indented. Then they again used the transmission x-ray microscope to image the wire to see how the internal structure had deformed.
When they examined the deformation, the researchers found something intriguing: the larger nanoparticles, thicker than ~80 nm, tended to buckle and kink when stressed. This buckling gave the alloy ductility as it allowed energy to dissipate. Meanwhile, the smaller nanoparticles of Al2Cu were what gave the material strength by serving as obstacles for slip in the material. Without these obstacles, stressing the aluminum caused the metal to separate into regions that slide against each other, eventually shearing apart. But the small, needle-like nanoparticles blocked those movements and prevented the shearing.
Typically, materials that are strong are also brittle, while ductile materials tend to be soft. It was thought that ductility and strength were mutually exclusive, but the results of this study suggest that aluminum alloys might be able to have both strength and ductility—if the distribution of small and large nanoparticles can be tuned just right. With clever metallurgy, we might be able to have aluminum alloys that are both super strong and ductile, the researchers suggest.
The next steps in this research will use the same beamline at the APS to examine how even smaller nanoparticles, as small as 20-nm in diameter, affect deformation in aluminum alloys. The researchers also plan to see if other elements such as magnesium and zinc form nanoparticles and affect aluminum alloy properties in ways similar to copper. The ultimate goal will be to understand the alloys’ behavior at the nanoscale well enough to design materials that are stronger and more durable than anything currently available. — Kim Krieger
See: C. Shashank Kaira1, Tyler J. Stannard1, Vincent De Andrade2, Francesco De Carlo2, and Nikhilesh Chawla1*, “Exploring novel deformation mechanisms in aluminum-copper alloys using in situ 4D nanomechanical testing,” Acta Mater. 176, 242 (2019). DOI: 10.1016/j.actamat.2019.07.016
Author affiliations: 1Arizona State University, 2Argonne National Laboratory
Correspondence: * nchawla@asu.edu
The authors are grateful for financial support from the Army Research Office under Contract No. W911NF1410550 (Dr. Michael Bakas and Dr. David Stepp, Program Managers). We acknowledge the use of facilities within the Center for 4D Materials Science and the Leroy Eyring Center for Solid State Science at Arizona State University. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
The APS is one of the world’s most productive x-ray light source facilities. Each year, the APS provides high-brightness x-ray beams to a diverse community of more than 5,000 researchers in materials science, chemistry, condensed matter physics, the life and environmental sciences, and applied research. Researchers using the APS produce over 2,000 publications each year detailing impactful discoveries, and solve more vital biological protein structures than users of any other x-ray light source research facility. APS x-rays are ideally suited for explorations of materials and biological structures; elemental distribution; chemical, magnetic, electronic states; and a wide range of technologically important engineering systems from batteries to fuel injector sprays, all of which are the foundations of our nation’s economic, technological, and physical well-being.
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC, for the U.S. DOE Office of Science.
The U.S. Department of Energy's Office of Science is the single largest supporter of basic research in the physical sciences in the United States and is working to address some of the most pressing challenges of our time. For more information, visit the Office of Science website.
